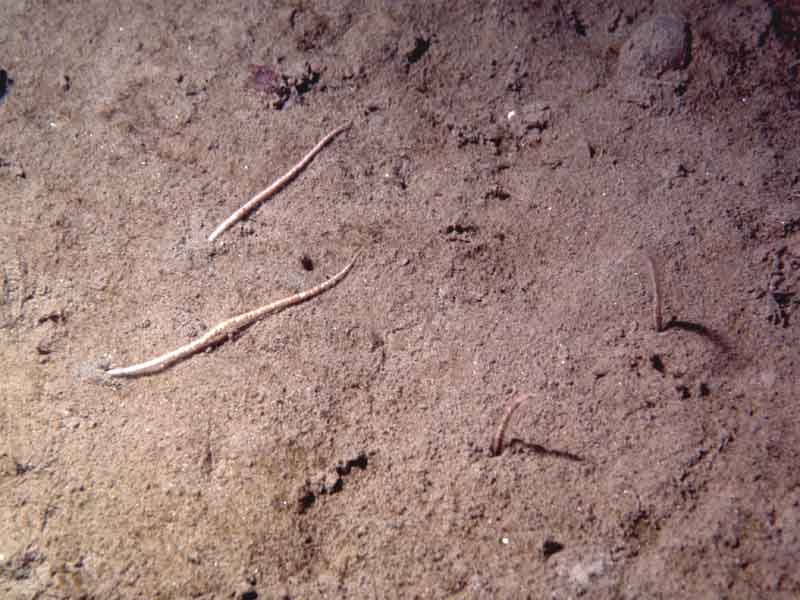
Amphiura filiformis, Kurtiella bidentata and Abra nitida in circalittoral sandy mud
| Researched by | Eliane De-Bastos, Jacqueline Hill & Amy Watson | Refereed by | Admin |
|---|
Summary
UK and Ireland classification
Description
Cohesive sandy mud off wave exposed coasts with weak tidal streams can be characterized by super-abundant Amphiura filiformis with Kurtiella bidentata (syn. Mysella bidentata) and Abra nitida. This community occurs in muddy sands in moderately deep water (Hiscock 1984; Picton et al., 1994) and may be related to the 'off-shore muddy sand association' described by other workers (Jones, 1951; Thorson, 1957; Mackie, 1990) and is part of the infralittoral etage described by Glemarec. This community is also characterized by the sipunculid Thysanocardia procera and the polychaetes Nephtys incisa, Phoronis sp. and Pholoe sp., with cirratulids also common in some areas. Other taxa such as Nephtys hombergii, Echinocardium cordatum, Nucula nitidosa, Callianassa subterranea and Eudorella truncatula may also occur in offshore examples of this biotope (e.g. Knitzer et al., 1992). (Information taken from Connor et al., 2004).
Depth range
10-20 m, 20-30 mAdditional information
-
Listed By
Habitat review
Ecology
Ecological and functional relationships
- The characterizing and other species in this biotope occupy space in the habitat but their presence is most likely primarily determined by the occurrence of a suitable substratum rather by interspecific interactions. Amphiura filiformis and Echinocardium cordatum are functionally dissimilar and are not always necessarily associated with each other but occur in the same muddy sediment habitats. There is no information regarding possible interactions between these species. In addition to Amphiura filiformis and Echinocardium cordatum, the biotope supports a fauna of burrowing species such as Callianassa subterranea and smaller less conspicuous species, such as polychaetes, nematodes and bivalves, living within the sediment.
- There are however some interspecific relationships within the biotope. The bivalve Tellimya (=Montacuta) ferruginosa is a commensal of Echinocardium cordatum, and as many as 14 or more of this bivalve have been recorded with a single echinoderm. Adult specimens live freely in the burrow of Echinocardium cordatum, while the young are attached to the spines of the echinoderm by byssus threads (Fish & Fish, 1996). The amphipod crustacean Urothe marina (Bate) is another common commensal (Hayward & Ryland, 1995).
- Most of the species living in deep mud biotopes are generally cryptic in nature and not usually subject to predation. However, the arms of Amphiura filiformis are an important food source for demersal fish and Nephrops norvegicus providing significant energy transfer to higher trophic levels. Increased nutrients leading to eutrophication processes (increased primary production) may contribute to increase the accumulation of hydrophobic contaminants in Amphiura filiformis and their transfer to higher trophic levels (Gunnarsson & Skold, 1999). Evidence of predation on Virgularia mirabilis by fish seems limited to a report by Marshall & Marshall (1882 in Hoare & Wilson, 1977), where the species was found in the stomach of haddock. Observations by Hoare & Wilson (1977) suggest, however, that predation pressure on this species is low. Many specimens of Virgularia mirabilis lack the uppermost part of the colony which has been attributed to nibbling by fish. The sea slug Armina loveni is a specialist predator of Virgularia mirabilis.
- In their investigation of density dependent migration in Amphiura filiformis, Rosenberg et al. (1997) calculated in areas of high density of the species (3000 individuals per m2), the area of sediment at about 3 to 4cm depth covered by disks of Amphiura filiformis can be estimated as 22%. The capacity of such a density of brittle stars to displace sediment can be calculated at 0.18 m2 per hour. Thus, movement of Amphiura filiformis should generate a more or less continuous displacement of sediment and be of great significance to the biogeochemical processes in the sediment.
- The burrowing and feeding activities of Amphiura filiformis modify the fabric and increase the mean particle size of the upper layers of the substrata by aggregation of fine particles into faecal pellets. Such actions create a more open fabric with a higher water content which affects the rigidity of the seabed (Rowden et al., 1998). Such destabilization of the seabed can affect rates of particle resuspension. At a permanent monitoring station in Galway Bay, the brittle star Amphiura filiformis consistently ranks third among the numerically dominant species. On this basis and due to its effect on the sediment (Ocklemann & Muus, 1978), it is tentatively given 'keystone' status within the community in question (O'Conner et al., 1983).
- The openings of the burrows of Callianassa subterranea provide temporary refuge for fish such as the black goby Gobius niger and the sand goby Pomatoschistus minutus. Occasional errant polychaetes, particularly polynoid worms, inhabit the burrows (Nickell & Atkinson, 1995).
- The bioturbatory activities of thalassinidean mud-shrimps such as Callianassa subterranea have important consequences for the structural characteristics of the sediment they inhabit.
- The hydrodynamic regime, which in turn controls sediment type, is the primary physical environmental factor structuring benthic communities such as CMS.AfilEcor. The hydrography also affects the water characteristics in terms of salinity, temperature and dissolved oxygen. It is also widely accepted that food availability (see Rosenberg, 1995) and disturbance, such as that created by storms (see Hall, 1994), are also important factors determining the distribution of species in benthic habitats.
Seasonal and longer term change
- Species such as Amphiura filiformis and Echinocardium cordatum are long-lived and are unlikely to show any significant seasonal changes in abundance or biomass. The numbers of some of the other species in the biotope may show peak abundances at certain times of the year due to seasonality of breeding and larval recruitment.
- Burrowing activity of the mud shrimp Callianassa subterranea in the North Sea appears to be seasonal (Rowden & Jones, 1997). Relatively little activity was observed in the period January - April, before a steady increase through spring and early summer, reaching a maximum at the end of the summer and a decline in autumn and winter. In January, when bioturbatory activity was low the seabed appeared essentially flat and smooth , whilst in September the bed was littered with numerous mounds and depressions.
- One of the key factors affecting benthic habitats is disturbance, which in shallow subtidal habitats will increase in winter due to weather conditions. Storms may cause dramatic changes in distribution of macro-infauna by washing out dominant species, opening the sediment to recolonization by adults and/or available spat/larvae (Eagle, 1975; Rees et al., 1977; Hall, 1994) and by reducing success of recruitment by newly settled spat or larvae (see Hall, 1994 for review). For example, during winter gales along the North Wales coast (Rees et al., 1976) northerly gales threw piles of Echinocardium cordatum on to the strand line and the author suggests these events are not uncommon. Lawrence (1989) also reports that Echinocardium cordatum and other organisms such as bivalves and brittlestars can be washed out of the sediment by water currents generated by gales.
Habitat structure and complexity
- The biotope has very little structural complexity with most species living in or on the sediment. The sediment expelled by Callianassa subterranea forms unconsolidated volcano-like mounds, which can significantly modify the seabed surface topography (Rowden et al., 1998). The sea pen, Virgularia mirabilis, and the anemone Cerianthus lloydii extend above the sediment surface, although these do not occur in high numbers and apart from a couple of species of nudibranch living on the sea pens, these species do not provide significant habitat for other fauna.
- Some structural complexity is provided by animal burrows although these are generally simple. The burrows of Echinocardium cordatum, for example, provide a habitat for other species such as the small bivalve Tellimya (=Montacuta) ferruginosa. Most species living within the sediment are limited to the area above the anoxic layer, the depth of which will vary depending on sediment particle size and organic content. The mud shrimp Callianassa subterranea creates complex burrow systems consisting of a multi-branched network of tunnels connected to several inhalant shafts, each terminating in a funnel shaped opening to the surface. The presence of burrows of species such as Echinocardium cordatum and Callianassa subterranea allows a much larger surface area of sediment to become oxygenated, and thus enhance the survival of a considerable variety of small species (Pearson & Rosenberg, 1978). Burrows also create habitats for other animals such as clams and polychaetes.
- Deposit feeders manipulate, sort and process sediment particles which may result in destabilization and bioturbation of the sediment which inhibits survival of suspension feeders. This can result in a change in the vertical distribution of particles in the sediment that may facilitate vertical stratification of some species with particle size preferences. Vertical stratification of species according to sediment particle size has been observed in some soft-sediment habitats (Peterson, 1977).
Productivity
Productivity in subtidal sediments is often quite low. Macroalgae are absent from CMS.AfilEcor and so productivity is mostly secondary, derived from detritus and organic material. Allochthonous organic material is derived from anthropogenic activity (e.g. sewerage) and natural sources (e.g. plankton, detritus). Autochthonous organic material is formed by benthic microalgae (microphytobenthos e.g. diatoms and euglenoids) and heterotrophic micro-organism production. Organic material is degraded by micro-organisms and the nutrients are recycled. The high surface area of fine particles provides surface for microflora. However, the arms of Amphiura filiformis are an important food source for demersal fish and Nephrops norvegicus, providing significant energy transfer to higher trophic levels.
Recruitment processes
- Studies of Amphiura filiformis suggest autumn recruitment (Buchanan, 1964) and spring and autumn recruitments (Glémarec, 1979). Using a 265µm mesh size, Muus (1981) identified a peak settlement period in the autumn with a maximum of 6800 recruits per m2. Muus (1981) shows the mortality of these settlers to be extremely high with less than 5% contributing to the adult population in any given year. In Galway Bay populations, small individuals make up ca. 5% of the population in any given month, which also suggests the actual level of input into the adult population is extremely low (O'Connor et al., 1983). The species is thought to have a long pelagic life so recruitment can come from distant sources.
- In Echinocardium cordatum the sexes are separate and fertilization is external, with the development of a pelagic larva (Fish & Fish, 1996). The fact that Echinocardium cordatum is to be found associated with several different bottom communities would indicate that the larvae are not highly selective and discriminatory and it is probable that the degree of discrimination in 'larval choice' becomes diminished with the age of the larvae (Buchanan, 1966). Metamorphosis of larvae takes place within 39 days after fertilization (Kashenko, 1994). On the north-east coast of England a littoral population bred for the first time when three years old. In the warmer waters of the west of Scotland breeding has been recorded at the end of the second year (Fish & Fish, 1996). Buchanan (1967) observed that offshore populations were very slow growing and did not appear to reach sexual maturity, so recruitment may be sporadic in places. However, since Buchanan (1967) also found that intertidal populations bred every year, recruitment should take place on an annual basis.
- Many of the other species in the biotope, including Callianassa subterranea and Virgularia mirabilis appear to have planktonic larvae so much recruitment to the biotope may be from distant sources.
Time for community to reach maturity
No evidence on community development was found. However, the two key species Amphiura filiformis and Echinocardium cordatum are long lived and take a relatively long time to reach reproductive maturity. It takes approximately 5-6 years for Amphiura filiformis to grow to maturity so population structure will probably not reach maturity for at least this length of time. In addition, Muus (1981) shows the mortality of new settling Amphiura filiformis to be extremely high with less than 5% contributing to the adult population in any given year. In Galway Bay (O'Connor et al., 1983) populations, small individuals make up ca. 5% of the population in any given month, which also suggests the actual level of input into the adult population is extremely low. Echinocardium cordatum breed for the first time when two to three years old. Recruitment of Echinocardium cordatum is often sporadic with reports of recruiting in only 3 years over a 10 year period (Buchanan, 1966), although this relates to subtidal populations. Intertidal individuals reproduce more frequently. Many of the other species in the biotope, such as polychaetes and bivalves, are likely to reproduce annually. However, because the key species in the biotope, Amphiura filiformis and Echinocardium cordatum, are long lived and take several years to reach maturity, the time for the overall community to reach maturity is also likely to be several years.
Additional information
-Preferences & Distribution
Habitat preferences
| Depth Range | 10-20 m, 20-30 m |
|---|---|
| Water clarity preferences | No information |
| Limiting Nutrients | No information |
| Salinity preferences | Full (30-40 psu) |
| Physiographic preferences | |
| Biological zone preferences | Circalittoral |
| Substratum/habitat preferences | Sandy mud |
| Tidal strength preferences | Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Exposed, Moderately exposed |
| Other preferences |
Additional Information
Species composition
Species found especially in this biotope
Rare or scarce species associated with this biotope
-
Additional information
Sensitivity review
Sensitivity characteristics of the habitat and relevant characteristic species
SS.SMu.CSaMu.AfilMysAnit, SS.SMu.CSaMu.ThyNten, SS.SMu.CSaMu.AfilNten, SS.SMu.OMu.LevHet, SS.SMu.OMu.PjefThyAfil and SS.SMu.OMu.MyrPo are circalittoral biotopes characterized by low energy hydrographic conditions that allow the development of stable sandy muds, and support rich and diverse infaunal communities. The biotopes SS.SMu.OMu.PjefThyAfil, SS.SMu.CSaMu.ThyNten, SS.SMu.CSaMu.AfilMysAnit, SS.SMu.CSaMu.AfilNten and SSA.OfusAfil, may comprise the Amphiura dominated components of the 'off-shore muddy sand association' (Jones, 1951; Mackie, 1990) and the infralittoral étage described by Glemarec (1973, cited in Connor et al., 2004). Little evidence on the polychaete fauna of the offshore sandy mud biotopes SS.SMu.OMu.LevHet, SS.SMu.OMu.PjefThyAfil and SS.SMu.OMu.MyrPo was found.
Therefore, the sensitivity of these Amphiura dominated biotopes is assessed as a group, on the assumption that their sensitivity is very similar in terms of substratum and functional groups present. Any differences in species or biotope response to pressures are highlighted. Although the biotopes also support diverse communities of other polychaete worms, bivalves, echinoderms and others, which contribute to species richness and diversity, these are not considered important characterizing, defining or structuring species and are not considered within the assessments. More information on these species can be found in other biotope assessments available on this website.
SS.SMu.CSaMu.AfilMysAnit occurs on cohesive sandy muds off wave exposed and moderately exposed deep waters with weak tidal streams. The biotope is characterized by super-abundant Amphiura filiformis with Kurtiella bidentata (syn. Mysella bidentata) and Abra nitida (Connor et al., 2004).
SS.SMu.CSaMu.ThyNten also occurs on cohesive sandy muds with small quantities of gravel, off sheltered or moderately exposed coasts with very weak tidal streams. The biotope may support populations characterized by Thyasira spp. and in particular Thyasira flexuosa, which may occur with Ennucula tenuis (syn. Nuculoma tenuis). Whilst moderately diverse, animal abundances are often low and it is possible that the biotope is the result of sedimentary disturbance e.g. from trawling, becoming an impoverished version of SS.SMu.CSaMu.AfilNten (Connor et al., 2004).
SS.SMu.CSaMu.AfilNten occurs in cohesive and non-cohesive sandy muds, off moderately exposed coasts in deep waters with very weak tidal streams. The biotope supports dense populations of Amphiura filiformis with the bivalve Ennucula tenuis (syn. Nuculoma tenuis) (Connor et al., 2004).
SS.SMu.OMu.LevHet, SS.SMu.OMu.PjefThyAfil and SS.SMu.OMu.MyrPo occur in deep offshore muds and sandy muds, and communities are characterized by the polychaetes Levinsenia gracilis and Heteromastus filiformis; polychaete Paramphinome jeffreysii, bivalves such as Thyasira spp. and the brittlestar Amphiura filiformis; and bivalve Myrtea spinifera with infaunal polychaetes respectively. However, no records have been found of these biotopes so the sensitivity assessments are largely based upon the description of the biotopes given by Connor et al. (2004).
Resilience and recovery rates of habitat
Amphiura filiformis is a small brittlestar, disc up to 10 mm in diameter, with very long arms (10x disc diameter) which lives buried in muddy sand. Muus (1981) showed the mortality of new settling Amphiura filiformis to be extremely high with less than 5% contributing to the adult population in any given year. Sköld et al. (1994) also commented on the high mortality and low rates of recruitment in this species. In Galway Bay populations (O'Connor et al., 1983), small individuals make up ca 5% of the population in any given month, which also suggests the actual level of input into the adult population is extremely low. Muus (1981) estimated the lifespan of Amphiura filiformis to be 25 years based on oral width (which does not change with gonadal growth) with recruitment taking place at the 0.3 mm disc size. In very long-term studies of Amphiura filiformis populations in Galway Bay, a lifespan of some 20 years is possible (O'Connor et al., 1983). Amphiura filiformis reaches sexual maturity after 2 years, breeds annually and, in the UK, one period of recruitment occurs in the autumn (Pedrotti, 1993). The species is thought to have a long pelagic life. Sköld et al. (1994) estimated the time lag between full gonads and settlement to be 88 days. This duration is comparable to the time period when pelagic larvae have been recorded in the plankton from July to November in one prior study, and August to December in another prior study (Fosshagen, 1965; Thorson, 1946, respectively, cited in Sköld et al., 1994). A long planktonic life stage means this species is predicted to disperse over considerable distances.
Kurtiella bidentata is a very small bivalve, up to 3 mm in length (Carter, 2008). The bivalve is often found in muddy sand or fine gravel, and associated with other species (e.g. brittlestar Acrocnida brachiata and other ophiurids) (Ockelmann & Muus, 1978; Carter, 2008). This is a viviparous species, with larvae retained in the gill pouch until an early shelled veliger stage, which then live for some time in the sea, common in summer and autumn (Lebour, 1938). Kurtiella bidentata produce planktonic larvae during a prolonged spawning season (Larsen et al., 2007), so are considered to have a high dispersal potential. Recruitment occurred during August-October and three year-classes were identified (O'Foighil et al., 1984). It is not known at what age this species becomes sexually mature, although both males and hermaphrodites can be found in their first year (Marshall, 2005). Kurtiella bidentata (studied as Mysella bidentata) was reported to be fast growing and have a lifespan of 5-6 years in the North Sea (Künitzer, 1989).
Abra nitida is considered to be an opportunistic bivalve species (Josefson, 1982) capable of exploiting newly disturbed substratum through larval recruitment, secondary settlement of post-metamorphosis juveniles, or redistribution of adults (Rees & Dare, 1993). Abra nitida recruitment tends to be episodic and may be negatively affected by the presence of predators and inhibited by high densities of adults (Josefson, 1982). Abra nitida has a larval planktonic phase indicating a high dispersal potential. In addition to dispersal via the plankton, dispersal of post-settlement juveniles in Abra spp. may occur via byssus drifting (Sigurdsson et al., 1976) and probably bed load transport (Emerson & Grant, 1991). Usually this species occurs in dense aggregations that undergo subsequent decline and then recover through dense settlement (Josefson, 1982).
Little information was available for bivalve Thyasira flexuosa. The larval development of the congener Thyasira equalis is lecithotrophic and the pelagic stage is very short or suppressed (Tillin & Tyler-Walters, 2014). This agrees with the reproduction of other Thyasira sp., and in some cases (e.g. Thyasira gouldii) no pelagic stage occurs at all (Thorson 1946, 1950). This means that larval dispersal is limited. Sparks-McConkey & Watling (2001) found that a population of Thyasira flexuosa in Penobscot Bay, Maine recovered rapidly (within 3.5 months) following trawler disturbance that resulted in a decrease in the population. Benthic reproduction allows re-colonization of nearby disturbed sediment and leads to rapid recovery where a large proportion of the population remains to re-populate the habitat.
Ennucula tenuis (syn. Nuculoma tenuis) is a small bivalve typically 1-2 cm in length and is free-living within sediments (MES, 2010). Harvey & Gage (1995) investigated reproduction and recruitment of the species from the Loch Etive, Scotland. They observed that synchronized spawning occurred in the winter, although no recruitment peak was evident, with benthic post-larvae present throughout the year. The authors also noted that there was spatial segregation occurring between adults and post-larvae, and suggested that high densities of adults could inhibit successful settlement and growth of post-larvae. Spawning of nuculids was restricted to a few months of the year (Harvey & Gage, 1995), and appeared to be controlled by endogenous factors, as well as environmental factors, such as temperature, salinity, light, tidal period and food available. In Nucula nitidosa from the German Bight, the timing of spawning in the summer and autumn was attributed to the seasonal rise in temperature during the summer months. At Plymouth, however, the same species appeared to breed in winter when bottom temperatures are falling, as is the case in Pronucula tenuis from Loch Etive (Harvey & Gage, 1995). The availability of a suitable food supply during the months prior to spawning may be a more potent determinant of spawning time (Berry, 1989; Tyler et al., 1992, both cited in Harvey & Gage, 1995), with annual variation in the availability and quality of food determining the exact time of spawning in any one year. The remaining evidence is based on related species Nucula nitidosa. The lifespan of Nucula nitidosa ranges from 7-10 years (Wilson, 1992). It takes 2-3 years for Nucula nitidosa to reach sexual maturity (Davis & Wilson, 1983b), and reproduce in high numbers. Once hatched, Nucula nitidosa larvae spend a short time in the water column (a few days), which reduces the risk of predation. However, juveniles do not have a high dispersal potential as they settle in the vicinity of the adults (Thorson, 1946).
Levinsenia gracilis tends to be found in deep water so little is known about the species. Levinsenia gracialis is a polychaete of the small Paraonidae family, which are known to be gonochoric. Larvae have been found in the plankton with up to 60 segments (Bhaud, 1983, cited in Rouse & Pleijel, 2001). This family is found in almost all deep-water regions of the world, and are only found on the surface of sandy or silty sediments or burrowing into the deeper layers of such sediments. Individuals tend to be non-selective surface or burrowing deposit feeders (Rouse & Pleijel, 2001).
Heteromastus filiformis is a medium-sized tube-dwelling polychaete belonging to the family Capitellidae. The body length is about 10 cm and the worm lives in a vertical tube extending to a depth of about 15 cm into muddy sands (Shaffer, 1983). Heteromastus filiformis has a lifespan of 2 years and reproduces once within 2 years. Several authors (cited in Shaffer, 1983) have suggested that it reproduces in spring. Lo Bianco (1909) in Italy and Fauvel (1927) in France reported that breeding and spawning occurred from September to April. Linke (1939), in the Bay of Jadebusen, Germany and Rasmussen (1956) in the Isefjord, Denmark both observed spawning in spring. Cazaux (1970) reported breeding and spawning in the early summer in the Bay of Arcahon, France. In the North Atlantic Ocean to the North Sea, recruitment also appears to be in spring (Gillet & Gorman, 2002). After spawning, the eggs are fertilized externally and released as a planktotrophic larva that spends up to 4 months in the plankton (Shaffer, 1983). Settlement is generally from April-May. Little is known of the fecundity of this genus. The planktonic larval phase allows significant recolonization from surrounding deposits, and the short lifespan allows relatively rapid restoration of biomass following colonization. Buchanan & Warwick (1974) concluded that Heteromastus filiformis spawned at the end of its second year, sometime between January and April, off the coast of England, followed by high mortality. Predators have a large effect on the mortality rate of Heteromastus juveniles, but not on the adults, and disturbance has a moderate effect on juvenile mortality (Shaffer, 1983).
No information regarding the longevity and life cycle of Paramphionome jeffreysii or Myrtea spinifera was found. Paramphionome jeffreysii is a polychaete of the Amphimonidae sub-family, which mainly occur in warm littoral waters. The group is known to be gonochoric with external fertilization. As far as it is known, the group are slow active predators, mainly on sessile animals, such as sponges, cnidarians, hydroids and ascidians (Rouse & Pleijel, 2001).
Myrtea spinifera is a small bivalve up to 2.5 cm long found in mud and muddy sands from the western coasts from south Devon to Shetland Isles, rarely elsewhere but is also recorded from Norway to Mediterranean (Hayward & Ryland, 1995b). Gamete production in most bivalves seems to involve the planktonic larvae strategy, characterized by high fecundity and high metabolic cost (Vance, 1973; Bayne, 1976 cited in Dame, 1996), with bivalves often considered as having variable recruitment success, likely to vary with environmental conditions.
Resilience assessment: Recovery of habitats following a disturbance is dependent on physical, chemical and biological processes and can be a more rapid process than in other areas (Bishop et al., 2006; cited in Fletcher et al., 2011). However, recovery times after physical disturbance have been found to vary for different sediment types (Roberts et al., 2010). Dernie et al. (2003) found that muddy sand habitats had the longest recovery times, compared to mud and clean sand habitats. Population recovery rates will be species specific. Removal of the characterizing species would result in the biotopes being lost and/or reclassified. Furthermore, these are stable biotopes, unlikely to be adapted to deal with disturbance. Brittlestar Amphiura filiformis is able to repair arms, has long dispersal potential, but is slow growing and takes two years to reach maturity. Bivalves Kurtiella bidentata, Abra nitida, Thyasira spp., Myrtea spinifera have fragile shells that are vulnerable to damage, are thought to be slow growing, have high dispersal potential, but recruitment tends to be sporadic. The polychaete species, including Levinsenia gracilis, Heteromastus filiformis and Paramphinome jeffreysii are often characterized by short lifespans and likely to have high recovery rates. So where the majority of the population remain (resistance is High or Medium), and/or recruitment by adult mobility is possible, resilience is likely to be High for all biotopes under assessment. However, where recovery through juvenile recruitment is required, this may be low in places and are dependent on favourable hydrodynamic conditions that allow settlement of new recruits. Although polychaetes tend to have high recovery rates, the low energy environments where the biotopes occur are likely to slow the time for most species to re-established biomass and age structured populations. Therefore, where impacts remove a significant proportion of the population (resistance is Low or None), recovery is likely to be Medium (2-10 years). Given that no information was found for some of the characterizing species of these biotopes, confidence in this assessment is ‘Low’.
NB: The resilience and the ability to recover from human induced pressures is a combination of the environmental conditions of the site, the frequency (repeated disturbances versus a one-off event) and the intensity of the disturbance. Recovery of impacted populations will always be mediated by stochastic events and processes acting over different scales including, but not limited to, local habitat conditions, further impacts and processes such as larval-supply and recruitment between populations. Full recovery is defined as the return to the state of the habitat that existed prior to impact. This does not necessarily mean that every component species has returned to its prior condition, abundance or extent but that the relevant functional components are present and the habitat is structurally and functionally recognizable as the initial habitat of interest. It should be noted that the recovery rates are only indicative of the recovery potential.
Hydrological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Temperature increase (local) [Show more]Temperature increase (local)Benchmark. A 5°C increase in temperature for one month, or 2°C for one year. Further detail EvidenceThe characterizing species in these biotopes are widely distributed in the British Isles, north-east Atlantic and beyond, from Norway to the Mediterranean, west and South Africa (Hayward & Ryland, 1995b). However, Thyasira populations in the British Isles are restricted to areas where the bottom waters remain cool all year round (Jackson, 2007). Additionally, Paramphinome jeffreysii seems to reach its southerly limit in UK waters suggesting a possible susceptibility to a long-term rise in summer water temperatures (Tillin & Tyler-Walters, 2014), although the sub-family to which it belongs, seems to mainly occur in warm littoral waters (Rouse & Pleijel, 2001). On the other hand, it is has been suggested that growth rates of Heteromastus filiformis are very rapid in warmer environments, with no growth occurring during winter (Shaffer, 1983). Kröncke et al. (2011) reported an increase in abundance and regional changes in distribution of various species with a southern distribution in the North Sea in 2000, and suggested the changes were largely associated with an increase in sea surface temperature, primary production and, thus, food supply. The authors suggested that the increase of annual average temperature was of about 1.1°C. Amphiura filiformis was among the species observed to have decreased. In Galway Bay, long-term recordings of water temperature at a site of high density aggregations of Amphiura filiformis showed the species is subject to annual variations in temperature of about 10°C (O'Connor et al., 1983). Increases in temperature may affect growth and fecundity. Muus (1981) showed that juvenile Amphiura filiformis are capable of much higher growth rates in experiments with temperatures between 12 and 17°C. Temperature not only limits the spatial distribution of bivalves, but also is a major controlling factor in many physiological rate processes like feeding and growth (Dame, 1996). For example, no spawning occurred in June in the wild, but specimens of Pronucula tenuis (studied as Nucula tenuis) held in laboratory at elevated temperature of 23°C were observed to spawn in July (Rachor, 1976, cited in Harvey & Gage, 1995). Furthermore, Wilson (1981) investigated temperature tolerances of six bivalve species from Dublin Bay. The author concluded that species variations in tolerance to increased temperature varied seasonally and with distribution along tidal height. Lethal temperatures for all six bivalve species in the study varied greatly and were, in most cases, well above 20°C. The maximum sea surface temperatures around the British Isles rarely exceed 20°C (Hiscock, 1998). Kurtiella bidentata (studied as Mysella bidentata) was recorded in Kinsale Harbour at temperatures ranging from 7.7-18.8°C (O’Brien & Keegan, 2006), and Künitzer (1989) reported that the main factor affecting the growth rate of Kurtiella bidentata (studied as Mysella bidentata) was temperature. Sensitivity assessment: The characterizing species of the biotopes are widely distributed and likely to occur both north and south of the British Isles, where typical surface water temperatures vary seasonally from 4-19°C (Huthnance, 2010). No information was found on the maximum temperature tolerated by the characterizing species. Elevated temperatures may affect growth of some of the characterizing species, but no mortality is expected. It is therefore likely that the characterizing species are able to resist a long-term increase in temperature of 2°C. However, Thyasira spp. may suffer some mortality because of an acute increase in temperature. So resistance is therefore assessed as Medium (loss <25%) for SS.SMu.CSaMu.ThyNten, and High for the remaining biotopes. Resilience is likely to be High, so SS.SMu.CSaMu.ThyNten is considered to have Low sensitivity to an increase in temperature at the pressure benchmark level, whereas the remaining biotopes are considered Not Sensitive. | HighHelp | HighHelp | Not sensitiveHelp |
Temperature decrease (local) [Show more]Temperature decrease (local)Benchmark. A 5°C decrease in temperature for one month, or 2°C for one year. Further detail EvidenceThe characterizing species in these biotopes are widely distributed in the British Isles, north-east Atlantic and beyond, from Norway to the Mediterranean and west and South Africa (Hayward & Ryland, 1995b). However, Thyasira populations in the British Isles are restricted to areas where the bottom waters remain cool all year round (Jackson, 2007). Additionally, Paramphinome jeffreysii seems to reach its southerly limit in UK waters (Tillin & Tyler-Walters, 2014), although the sub-family to which it belongs, seems to mainly occur in warm littoral waters (Rouse & Pleijel, 2001). On the other hand, Heteromastus filiformis occur in the North Sea, English Channel, north-east Atlantic and Mediterranean (Hayward & Ryland, 1995b), and it has been suggested that no growth occurs during winter (Shaffer, 1983), suggesting intolerance to decreases in temperature. Holme (1967) reported the absence of Amphiura filiformis from samples taken from Weymouth Bay and Poole Bay, England, after severe winter temperatures (4 and 5°C, respectively, below the mean for about a month). In Galway Bay, long-term recordings of water temperature at a site of high density aggregations of Amphiura filiformis showed the species is subject to annual variations in temperature of about 10°C (O'Connor et al., 1983). However, echinoderms, including Amphiura filiformis, in the North Sea, seem periodically affected by winter cold. A population at 27 m depth off the Danish coast was killed by the winter of 1962-63 (Muus, 1981) and a population at 35-50 m depth in the inner German Bight was killed in the winter of 1969-1970 and a new population was not re-established until 1974 (Gerdes, 1977). Ursin (1960, cited in Gerdes, 1977) suggests that Amphiura filiformis does not occur in areas with winter temperatures below 4 °C although in Helgoland waters it can tolerate temperatures as low as 3.5 °C. Temperature not only limits the spatial distribution of bivalves, but also is a major controlling factor in many physiological rate processes like feeding and growth, with short-term acute periods of extreme cold and icing conditions considered likely to cause stress and some mortality in bivalve populations (Dame, 1996). For example, Kurtiella bidentata (studied as Mysella bidentata) was among the species that suffered high losses that could be related to low temperatures in the Wadden Sea area in 1979, where temperature was 3 degrees below average for 3 months (Beukema, 1979). During the 1978/79 winter, which was very cold with severe ice conditions, water temperature in the outer Weser estuary, Germany, remained below 0 °C on 45 successive days. Populations of the characteristic species of the benthos, including Abra spp. were considerably damaged (Buhr, 1981). Coyle et al. (2007) analysed temporal differences in benthic infaunal samples from the south-eastern Bering Sea shelf. Significant differences were observed for specific functional groups, namely carnivores, omnivores and surface detritivores, which suggested a mechanistic link between temperature changes and infaunal biomass, with exclusion of benthic predators on infaunal invertebrates by the cold bottom water on the shelf. In studies of the effects of cold winters on macrofauna communities in the North Sea, Kröncke et al. (2013) suggested that the overall trend was towards decreased abundance and biomass, including polychaetes, as a result of temperature anomalies of about 2°C below normal. However, Holte et al. (2005) investigated the variations in soft bottom macrofauna from stratified Norwegian basins. Heteromastus filiformis occurred at the study sites, which experienced temperatures between 0.5-14°C. Sensitivity assessment: The characterizing species of the biotope are widely distributed and likely to occur both north and south of the British Isles, where typical surface water temperatures vary seasonally from 4-19°C (Huthnance, 2010). Although it is likely that the characterizing species are able to resist a long-term decrease in temperature of 2°C, species may suffer some mortality as a result of an acute decrease in temperature. Therefore, resistance is assessed as Low (25-75% loss) and resilience is likely to be Medium, so the biotopes are considered to have Medium sensitivity to a decrease in temperature at the pressure benchmark level. | LowHelp | MediumHelp | MediumHelp |
Salinity increase (local) [Show more]Salinity increase (local)Benchmark. A increase in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidenceThe biotopes are found within fully marine subtidal locations (Connor et al., 2004). Therefore, it is highly unlikely that the biotopes would experience conditions of hypersalinity. Echinoderms, such as Amphiura filiformis, are stenohaline owing to the lack of an excretory organ and a poor ability to osmo- and ion-regulate (Stickle & Diehl, 1987; Russell, 2013). A review by Russell (2013) confirmed that none of the echinoderm species relevant in this assessment occur in hypersaline conditions. Pagett (1981) suggested that localised physiological adaption to reduced or variable salinities may occur in nearshore areas subject to freshwater runoffs. However, individuals in these biotopes are unlikely to experience variable salinities, and resident species unlikely to be adapted to variation in salinity, as suggested by the results given by Pagett (1981). The capitellid polychaete Heteromastus filiformis has been recorded in the Homa lagoon, eastern Aegean Sea, where salinity exceeded 40 psu all year round, and negative effects on the species density were reported as a result of salinity increases to 59-61.5 psu (Can et al., 2012), which is higher than the benchmark level. The minimum and maximum recorded ranges of salinities for the remaining characterizing species found are 18.6 - 38.6 pps for Kurtiella bidentata, 17.8 - 39.1 pps for Abra nitida, 31.8-39.1 pps for Thyasira flexuosa, 32.3 - 38.6 pps for Paramphinome jeffreysii, and 33.4 - 39.0 for Myrtea spinifera (OBIS, 2014). Similar data was not available for Levinsenia gracilis and Pronucula tenuis. Sensitivity assessment: There is little direct evidence of the effects of hypersaline conditions on the characterizing species of these biotopes. However, based on the information presented, all key species except for Heteromastus filiformis, are likely to suffer significant (25-75%) mortality as a result of an increase in salinity to >40 psu. Resistance is assessed as Low but with low confidence. Resilience is probably Medium, so sensitivity is therefore assessed as Medium. | LowHelp | MediumHelp | MediumHelp |
Salinity decrease (local) [Show more]Salinity decrease (local)Benchmark. A decrease in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidenceThe biotopes are found within fully marine subtidal locations (Connor et al., 2004). Therefore, it is highly unlikely that the biotopes would experience conditions of hyposalinity. Echinoderms, such as Amphiura filiformis, are stenohaline owing to the lack of an excretory organ and a poor ability to osmo- and ion-regulate (Stickle & Diehl, 1987; Russell, 2013). However, Amphiura filiformis was recorded in hyposaline conditions in the Sado estuary in Portugal (Monteiro-Marques, 1982 cited in Russell, 2013) where the salinity was 25.5‰, and in the Black Sea where it tolerated 8.9‰ (Russell, 2013). Pagett (1981) suggested that localised physiological adaption to reduced or variable salinities may occur in nearshore areas subject to freshwater runoffs. However, individuals in these biotopes are unlikely to experience variable salinities, and resident species are unlikely to be adapted to variation in salinity, as suggested by the results given by Pagett (1981). Salinity may affect the structural and functional properties of bivalve organisms through changes in total osmotic concentration, relative proportion of solutes, coefficients of adsorption and saturation of dissolved gases and density and viscosity (Kinne, 1964, cited in Dame, 1996). There are records of Kurtiella bidentata (studied as Mysella bidentata) in Kinsale Harbour at salinities ranging from 19.3-35.0 (O’Brien & Keegan, 2006). However, Gogina et al. (2010a) reported that Kurtiella bidentata (studied as Mysella bidentata) showed a strong positive correlation with salinity varying at a factor of 8.30-27.10 psu, which suggested that the species was affected adversely at the low end of the range. According to Budd (2007), the change would be likely to cause inhibition of growth and reproduction, and exposure to low salinity may result in some mortality of Abra spp.. Thyasira spp. inhabit waters of reduced salinity with 25-30 psu being optimal. However, adults exposed to lower than optimal salinities produced non-viable or slow developing eggs (Jackson, 2007). There is insufficient information regarding the effects of salinity on adults. Furthermore, the minimum and maximum recorded ranges of salinities for the characterizing species found are 18.6 - 38.6 pps for Kurtiella bidentata, 17.8 - 39.1 pps for Abra nitida, 31.8-39.1 pps for Thyasira flexuosa, 32.3 - 38.6 pps for Paramphinome jeffreysii, and 33.4 - 39.0 for Myrtea spinifera (OBIS, 2014), although the source of the data is unclear. Similar data was not available for Levinsenia gracilis and Pronucula tenuis. Sensitivity assessment: The evidence presented suggests that not all characterizing species of the biotopes are likely to resist a decrease in salinity at the pressure benchmark level. Resistance is therefore assessed as Low (loss of 25-75%) but with low confidence. Once prior conditions are resumed, resilience is probably Medium so sensitivity is therefore assessed as Medium. | LowHelp | MediumHelp | MediumHelp |
Water flow (tidal current) changes (local) [Show more]Water flow (tidal current) changes (local)Benchmark. A change in peak mean spring bed flow velocity of between 0.1 m/s to 0.2 m/s for more than one year. Further detail EvidenceThe hydrographic regime, including flow rates, is an important structuring factor in sedimentary habitats. The low energetic environments where the biotopes occur are therefore likely to be important in allowing for the development of the sandy mud substrata which characterize the biotopes. The most damaging effect of increased flow rate (above the pressure benchmark) would be the erosion of the substratum as this could eventually lead to loss of the habitat. Increased water flow rates are likely to change the sediment characteristics in which the species live, primarily by resuspending and preventing deposition of finer particles (Hiscock, 1983). Furthermore, increased water flow rate may prevent settlement of larvae and therefore reduce recruitment. Mature adults buried at depth are likely to be unaffected as muddy substrata are particularly cohesive. Additionally, the consequent lack of deposition of particulate matter at the sediment surface would reduce food availability. Decreased water movement would result in increased deposition of suspended sediment (Hiscock, 1983). An increased rate of siltation resulting from a decrease in water flow may result in an increase in food availability for the characterizing species and therefore growth and reproduction may be enhanced, but only if food was previously limiting. Nevertheless, a decrease in water flow rates is unlikely to be relevant in the low energy environments where the biotopes occur. Amphiura filiformis respond rapidly to currents by extending their arms into the water column to feed. Under laboratory conditions, they were shown to maintain this vertical position at currents of 0.3 m/s (Buchanan, 1964). Amphiura filiformis feed on suspended material in flowing water but change to deposit feeding in stagnant water or areas of very low water flow (Ockelmann & Muus, 1978). Food requirements probably set a lower limit on the current regime of areas able to support brittlestars. Amphiura filiformis has also been reported in the Northumberland coast, UK where tidal currents ranged, with surface speeds of 0.65 m/s at springs to 0.4 m/s at neaps, on a flood tide. Bottom residual currents were much weaker than near-surface, reaching a maximum of 0.7 m/s (Jones, 1979, cited in Birchenough & Frid, 2009). Sensitivity assessment: Sand particles are most easily eroded and likely to be eroded at about 0.20 m/s (based on Hjulström-Sundborg diagram, Sundborg, 1956). Although having a smaller grain size than sand, silts and clays require greater critical erosion velocities because of their cohesiveness. The biotopes occur in stable areas of very weak (negligible) and weak (>0.5 m/s) tidal streams (Connor et al., 2004). Although changes in water flow (above the benchmark) would be likely to change the sedimentary regime in the biotopes, the cohesive nature of the sandy muds that characterize the biotopes is likely to provide some protection to changes in water flow at the pressure benchmark. Additionally, the characterizing species are likely to resist an increase in water flow at the benchmark level. Resistance and resilience are, therefore, assessed as High and the biotopes considered Not Sensitive to a change in water flow at the pressure benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Emergence regime changes [Show more]Emergence regime changesBenchmark. 1) A change in the time covered or not covered by the sea for a period of ≥1 year or 2) an increase in relative sea level or decrease in high water level for ≥1 year. Further detail EvidenceThe biotopes are circalittoral (Connor et al., 2004). Changes in emergence are Not Relevant to biotopes which are restricted to fully subtidal/circalittoral conditions. The pressure benchmark is relevant only to littoral and shallow sublittoral fringe biotopes. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Wave exposure changes (local) [Show more]Wave exposure changes (local)Benchmark. A change in near shore significant wave height of >3% but <5% for more than one year. Further detail EvidencePotentially the most damaging effect of increased wave heights would be the erosion of the fine sediment substratum as this could eventually lead to loss of the habitat that characterizes the biotopes. Decreased exposure will probably lead to increased siltation and reduced grain size (muddy sediment). Changes in wave exposure may therefore influence the supply of particulate matter for tube building and feeding activities of the characterizing species. Food supplies may also be reduced affecting growth and fecundity of the species. Strong wave action may cause damage or withdrawal of the siphons and delicate feeding structures, resulting in loss of feeding opportunities and compromised growth for the characterizing species. Additionally, individuals may be dislodged by scouring from sand and gravel mobilized by increased wave action (Budd, 2007). During winter gales along the North Wales coast, large numbers of Abra spp. were cast ashore and over winter survival rate was as low as 7% in the more exposed locations (Rees et al., 1977). Additionally, changes in wave exposure may also interfere with larval dispersal of the characterizing species. Olivier et al. (1996) reported that the post-larvae and juveniles of Abra alba were most abundant in the near-bottom water stratum at flood tides. Therefore, increased wave action could result in enhanced resuspension and dispersal of early life stages, whereas a reduction in wave exposure may lead to a decrease in dispersal. Amphiura filiformis is found in sheltered habitats characterized by fine muddy sandy sediments and low wave exposure. The species is unlikely to be resistant of increases in wave exposure because strong wave action can resuspend the sediment and break up and scatter Amphiura filiformis. However, the species is able to burrow further into the sediment and if displaced is able to reburrow (Hill & Wilson, 2008). Thyasira gouldii lives in rather wave sheltered areas at the heads of sea lochs (Jackson, 2007). Increases in wave exposure may disrupt the sediment in which they live, cause continual displacement and physical damage to the shells which are thin and fragile. Sensitivity assessment: No direct evidence of the specific tolerances of the characterizing species to changes in wave exposure was found. Hiscock (1983) suggested that a Force 8 Gale could result in oscillatory wave induced water flow at 80 m of 0.09 m/s or ca 0.4 m/s at 50 m. A change in significant wave height of 3-5% is roughly equivalent to a change from force 3-4. Therefore, it is unlikely to be significant in deep water biotopes. Except for SS.SMu.CSaMu.AfilMysAnit, the biotopes under assessment occur at depths (>20 m) which are likely to protect the biotopes from anything other than the most severe change in wave action. However, SS.SMu.CSaMu.AfilMysAnit is the least sheltered of the biotopes, occurring in exposed and moderately conditions (Connor et al., 2004), and a change at the benchmark level is likely to fall within the range experienced by this particular biotope. Resistance and resilience are, therefore, assessed as High, and the biotopes are considered Not Sensitive at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Chemical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Transition elements & organo-metal contamination [Show more]Transition elements & organo-metal contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed but evidence is presented where available. There is little or no information on the resistance of the characteristic species in the biotopes. Experimental studies with various species suggest that polychaete worms are quite tolerant of heavy metals (Bryan, 1984). Bryan (1984) also reports that early work has shown that echinoderm larvae are intolerant of heavy metals, e.g. the intolerance of larvae of sea urchin Paracentrotus lividus to copper (Cu) had been used to develop a water quality assessment. Adult echinoderms are known to be efficient concentrators of heavy metals including those that are biologically active and toxic (Hutchins et al., 1996). However, there is no information available regarding the effects of this bioaccumulation. Studies by Deheyn & Latz (2006) at the Bay of San Diego found that heavy metal accumulation in brittlestars occurs both through dissolved metals as well as through diet, to the arms and disc, respectively. Similarly, Sbaihat et al. (2013) measured concentrations of heavy metals (Cu, Ni, Cd, Co, Cr and Pb) in the body of Ophiocoma scolopendrina collected from the Gulf of Aqaba, and found that most concentration was found in the central disc rather than arms and no simple correlations could be found between contaminant and body length. Abra spp. can live in polluted sediments (Dauvin, pers. comm.), for example, near Calais where high densities of Abra alba were found in sediment containing 8 mg/g iron and 4 mg/g titanium (Dewarumez et al., 1976). The capacity of bivalves to accumulate heavy metals in their tissues, far in excess of environmental levels, is well known. Bryan (1984) stated that Hg is the most toxic metal to bivalve molluscs while Cu, Cd and Zn seem to be most problematic in the field. In bivalve molluscs Hg was reported to have the highest toxicity, mortalities occurring above 0.1-1 g/l after 4-14 days exposure (Crompton, 1997), toxicity decreasing from Hg > Cu and Cd > Zn > Pb and As > Cr ( in bivalve larvae, Hg and Cu > Zn > Cd, Pb, As, and Ni > to Cr). | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Hydrocarbon & PAH contamination [Show more]Hydrocarbon & PAH contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed but evidence is presented where available. Invertebrate communities respond to severe chronic oil pollution in much the same way. Initial massive mortality and lowered community diversity is followed by extreme fluctuations in populations of opportunistic mobile and sessile fauna (Suchanek, 1993). Infaunal communities, such as those characterizing these biotopes are highly likely to be adversely affected by an event of oil pollution, but the biological effects of accumulation of PAHs are likely to depend on the length of time exposed (Viñas et al., 2009). Oil contamination is likely to remain in the sediment for a long time after the pollution source is removed. Ingestion of contaminated sediments is likely to be a more important route of exposure for deposit feeders such as the characterizing species of these biotopes. Echinoderms have not been found to be resistant to the toxic effects of oil, likely because of the large amount of exposed epidermis (Suchanek, 1993), and tend to be very sensitive to various types of marine pollution (Newton & McKenzie, 1995). In a study of the effects of oil exploration and production on benthic communities, Olsgard & Gray (1995) found Amphiura filiformis to be very intolerant of oil pollution. During monitoring of sediments in the Ekofisk oilfield, Addy et al. (1978) suggested that reduced abundance of Amphiura filiformis within 2-3 km of the site was related to discharges of oil from the platforms and to physical disturbance of the sediment. Brittlestars host symbiotic sub-cuticular bacteria (Kelly & McKenzie, 1995). After exposure to hydrocarbons, loadings of such bacteria were reduced indicating a possible sub-lethal stress to the host (Newton & McKenzie, 1995). Suchanek (1993) reviewed the effects of oil spills on marine invertebrates and concluded that, in general, on soft sediment habitats, infaunal polychaetes, bivalves and amphipods were particularly affected. Sub-lethal concentrations may produce substantially reduced feeding rates and/or food detection ability, probably due to ciliary inhibition. Respiration rates may increase at low concentrations and decrease at high concentrations. Generally, contact with oil causes an increase in energy expenditure and a decrease in feeding rate, resulting in less energy available for growth and reproduction. However, the Abra alba population affected by the 1978 Amoco Cadiz benefited from the nutrient enrichment caused by the oil pollution. The biomass of the fine-sand community remained low in 1979, a year after the spill, owing to the decimation of the Ampelisca amphipod population, but the biomass then doubled as a result of an increase in Abra alba abundance in 1980 and Abra alba remained a dominant species over the 20 year duration over which recovery of the community was monitored (Dauvin, 1998). Untreated oil (e.g. from oil spills) is not a risk, since it is concentrated mainly at the surface, and circalittoral biotopes are likely to be protected by their depth. If oil is treated by dispersant, the resulting emulsion may penetrate down the water column, especially under the influence of turbulence (Hartnoll, 1998). | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Synthetic compound contamination [Show more]Synthetic compound contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed but evidence is presented where available. Echinoderms tend to be very sensitive to various types of marine pollution (Newton & McKenzie, 1995) but there is no more detailed information than this broad statement. In laboratory experiments Smith (1968) found the concentration of BP1002 (the detergent used in the Torrey Canyon oil spill clean-up) needed to kill the majority of brittlestar Ophiocomina nigra was 5 ppm. Dahllöf et al. (1999) studied the long-term effects of tri-n-butyl-tin (TBT) on the function of a marine sediment system. TBT spiked sediment was added to a sediment that already had a TBT background level of approximately 27 ng/g (83 pmol TBT per g) and contained Amphiura spp., and several species of polychaete. Within two days of treatment with a TBT concentration above 13.7 µmol/m² all species except the polychaetes had crept up to the surface and after six weeks these fauna had started to decay. Thus, contamination from TBT is likely to result in the death of some non-resistant species such as brittlestars. However, Walsh et al. (1986) observed inhibition of arm regeneration in another brittlestar, Ophioderma brevispina, following exposure to TBT at levels between 10 ng/l and 100 ng/l. Loizeau & Menesguen (1993), found that 8-15% of the PCB burden in dab, Limanda limanda, from the Bay of Seine could be explained by ophiuroid consumption. Thus, Amphiura communities may play an important role in the accumulation, remobilization and transfer of PCBs and other sediment associated contamination to higher trophic levels. Abra spp. demonstrated alterations of its behaviour in response to exposure to marine sediments contaminated with pesticides (6000 ppm parathion, 200 ppm methyl parathion, 200 ppm malathion). No burrowing occurred in the most contaminated sediment, whilst burrowing was impaired in the moderately contaminated sediment with a median effective burrowing time (ET(50)) of 9.0 (±3.0 - 28) minutes in comparison to a control time of 4.5 (±2.8 - 7.2) minutes (Møhlenberg & Kiørboe, 1983). There is no evidence relating directly to the effects of synthetic chemicals on the remaining characterizing species. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Radionuclide contamination [Show more]Radionuclide contaminationBenchmark. An increase in 10µGy/h above background levels. Further detail EvidenceAdult echinoderms are known to be efficient concentrators of radionuclides (Hutchins et al., 1996). However, no information concerning the effects of such bioaccumulation was found. Carvalho (2011) determined the concentrations of 210Po and 210Pb in marine organisms from the seashore to abyssal depths, as these two radioactive elements tend to be higher in the marine environment. The author’s results showed that concentrations varied greatly, even between organisms of the same biota, mainly related with the trophic levels occupied by the species, suggesting that the more levels between a species and the bottom of the food chain, the more likely that the concentrations of radioactive elements were likely to be diluted. This may have great implications for the deposit feeders that characterize these biotopes. There was no information available about the effect of this bioaccumulation. Sensitivity assessment: There is no substantial evidence available on which to assess this pressure. The pressure is therefore assessed as No Evidence. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction of other substances [Show more]Introduction of other substancesBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
De-oxygenation [Show more]De-oxygenationBenchmark. Exposure to dissolved oxygen concentration of less than or equal to 2 mg/l for one week (a change from WFD poor status to bad status). Further detail EvidenceOxygen-deficient marine areas are characterized by a decline in the number and diversity of species. Cole et al. (1999) suggested possible adverse effects on marine species exposed to dissolved oxygen concentrations below 4 mg/l and probable adverse effects below 2 mg/l. A number of animals have behavioural strategies to survive periodic events of reduced dissolved oxygen. These include shell closure and reduced metabolic rate in bivalve molluscs and either decreased burrowing depth or emergence from burrows for sediment dwelling crustaceans, molluscs and annelids. However, a decrease in oxygenation is likely to see the loss of the key species in the biotopes. During periods of hypoxia infaunal species migrate to the surface of the sediment (Diaz & Rosenberg, 1995). Stachowitsch (1984) observed a mass mortality of benthic organisms in the Gulf of Trieste, northern Adriatic Sea, caused by the onset of severe hypoxia in the near-bottom water. A wide variety of organisms were affected, including burrowing invertebrates, sponges, and the brittlestar Ophiothrix quinquemaculata. However, Amphiura filiformis was reported as a species resistant to moderate hypoxia (Diaz & Rosenberg, 1995). In experiments exposing benthic invertebrates to decreasing oxygen levels, Amphiura filiformis only left its protected position in the sediment when oxygen levels fell below 0.85 mg/l, and was able to survive for several weeks (Rosenberg et al., 1991). This escape response increases predation risk. Mass mortality of Amphiura filiformis was observed during severely low oxygen events (<0.7 mg/l) (Nilsson, 1999). Mass mortality was observed following large increases in eutrophication and subsequent reductions in oxygen (Vistisen & Vismann, 1997). The regeneration rate of arms is significantly decreased at low oxygen concentrations (1.8-2.2 mg/l) (Nilsson, 1999), and growth rate is decreased in oxygen concentrations of <2.7 mg/l and spawning is restricted (Nilsson & Sköld, 1996). Infaunal burrowers in the community live in close association with hypoxic and even anoxic muddy substrata, including the characterizing polychaetes. Heteromastus filiformis was recorded to occur in the Homa lagoon, eastern Aegean Sea, where high salinity was coupled with low oxygen concentrations (2.3-3.9 mg/l) with adverse negative effects on the abundance of the community (Can et al., 2012). However, the study focused on the effects of hypersalinity (>50 psu) and the authors attributed mortality to increased salinity, leaving it unclear whether the hypoxic conditions also contributed to mortality of the population. This agrees with Hiscock et al. (2005a), who reported Heteromastus filiformis as a species resistant to severe hypoxia. At oxygen concentrations below ca 0.4 mg O2/l, Kurtiella bidentata eventually emerged from the substratum (Ockelmann & Muus, 1978). Nilsson & Rosenberg (1994) investigated hypoxic responses of benthic communities and reported Kurtiella bidentata (studied as Mysella bidentata) leaving the sediment at oxygen concentrations of 1.7 mg/l. According to the authors, this behaviour that occurs at hypoxic oxygen concentrations is slightly higher than those causing mortality, suggesting high levels of stress caused to the organisms. Abra spp. are typically found in organically enriched sediments where it may be present in high densities (Dauvin & Gentil, 1989). Experimental examination of the interactions between eutrophication and oxygen deficiency (2.4-3.5 mg O2/l over a 93 day experimental period) revealed that Abra alba became inefficient in its use of the available organic matter under prolonged conditions of hypoxia (Hylland et al., 1996). Abra alba was also reported to be sensitive to lowered oxygen concentrations off the Swedish west coast (Rosenberg & Loo, 1988; Weigelt & Rumohr, 1986, both cited in Rees & Dare, 1993). López-Jamar et al. (1987) stated that Thyasira flexuosa was adapted to living in reduced sediments and also was found in organically enriched sediments. However, Dando & Spiro (1993) found that numbers of the congeners Thyasira equalis and Thyasira sarsi decreased rapidly following the de-oxygenation of bottom water in the deep basin of the Gullmar fjord in 1979-80. In a meta-analysis study of hypoxia, median sub-lethal oxygen concentrations reported in experimental assessments. Although no specific data was reported for all the characterizing species of these biotopes, the thresholds of hypoxia for different benthic groups was LC50 1.42 mg/l for bivalves, and sub-lethal (SLC50) of 1.20 mg/l for annelids (Vaquer-Sunyer & Duarte, 2008). For Kurtiella bidentata (studied as Mysella bidentata), the median sub-lethal oxygen concentrations reported in experimental assessments was 1 mg/l, and for Abra spp. was 0.57 mg/l (Vaquer-Sunyer & Duarte, 2008). Sensitivity assessment: Cole et al. (1999) suggest possible adverse effects on marine species below 4 mg/l and probable adverse effects below 2 mg/l. Based on the evidence presented, the characterizing species are likely to only be affected by severe deoxygenation episodes. However, some mortality of Thyasira spp. might occur in near anoxic (0% oxygen) conditions. So resistance is therefore assessed as Medium (loss <25%) for SS.SMu.CSaMu.ThyNten, and High for the remaining biotopes. Resilience is likely to be High so SS.SMu.CSaMu.ThyNten is considered to have Low sensitivity to exposure to dissolved oxygen concentration of less than or equal to 2 mg/l for 1 week, whereas the remaining biotopes are considered Not Sensitive. | HighHelp | HighHelp | Not sensitiveHelp |
Nutrient enrichment [Show more]Nutrient enrichmentBenchmark. Compliance with WFD criteria for good status. Further detail EvidenceIncreased nutrients are most likely to affect abundance of phytoplankton which may include toxic algae (OSPAR, 2009). This primary effect resulting from elevated nutrients will affect other biological elements or features (e.g. toxins produced by phytoplankton blooms or de-oxygenation of sediments) and may lead to ‘undesirable disturbance’ to the structure and functioning of the ecosystem. With enhanced primary productivity in the water column, organic detritus that falls to the seabed may also be enhanced. Interface feeders such as Amphiura filiformis have been reported to respond rapidly to increased primary production that may result from increased nutrient availability (Pearson & Mannvik, 1998, cited in Schückel et al., 2010). In a sewage dumping region of the North Sea, a great increase in the abundance of Abra spp. occurred in much of the dumping area because of the ecological adaptations of the species enabled it to exploit the greatly increased supply of nutrients (Caspers, 1981). The Amoco Cadiz oil spill in March 1978 caused vast disturbance to the fine-sand communities of the Bay of Morlaix, France (Dauvin, 1982). Drastic qualitative and quantitative changes in species abundance, diversity, and biomass were recorded after the spill. However, the Abra alba population persisted in the disturbed environment under eutrophic conditions and, as an 'opportunistic species' (Hily & Le Bris, 1984), rapidly adapting its reproductive strategy to three spawnings per year. Increased growth and abundance were attributable to increased food availability and vacant ecological niches (Dauvin & Gentil, 1989). Enrichment from pulp mills is believed to have been the cause of the death of two populations of Thyasira spp. in west Scotland sea lochs. Thyasira flexuosa has been recorded at densities of up to 4000 per square metre in enriched areas (Jackson, 2007). In a report to identify seabed indicator species to support implementation of the EU habitats and water framework directives, Amphiura spp., Kurtiella bidentata, Levinsenia gracilis, Heteromastus filiformis and Thyasira spp. were reported as likely to be favoured by nutrient enrichment, whereas Abra spp. were assessed as intolerant (Hiscock et al., 2005a). Sensitivity assessment: The overall species diversity in these biotopes is likely to decline given the varying responses of the species occurring here to nutrient enrichment (Hiscock et al., 2005a). The community, and hence the biotopes, may change to one dominated by nutrient enrichment resistant species, in particular polychaete worms. However, these changes generally refer to gross nutrient enrichment. A decrease in nutrient availability may result in impaired growth and fecundity although species diversity is not likely to be affected significantly. Nevertheless, the biotopes are considered to be Not Sensitive at the pressure benchmark that assumes compliance with WFD good status. | Not relevant (NR)Help | Not relevant (NR)Help | Not sensitiveHelp |
Organic enrichment [Show more]Organic enrichmentBenchmark. A deposit of 100 gC/m2/yr. Further detail EvidenceOrganic enrichment is likely to promote pelagic productivity and increase the amount of organic matter reaching the seabed, which may be beneficial to deposit feeders as a direct source of food. Nilsson (1999) investigated the effects of organic enrichment (control 0 gC/m2, medium 27 gC/m2 and high 55 gC/m2) on arm regeneration of Amphiura filiformis over a two month period. Amphiura filiformis responded positively to increased organic enrichment (Nilsson, 1999). In the Skagerrak in the North Sea, a massive increase in abundance and biomass of the brittlestar between 1972 and 1988 was attributed to organic enrichment (Josefson, 1990; Hernroth et al., 2012). Rosenberg et al. (1997) also reported that Amphiura filiformis appeared to be more densely packed in the sediment when food occurred superabundantly compared to when food was less common. Sköld & Gunnarsson (1996) reported enhanced growth and gonad development in response to short-term enrichment of sediment cores containing Amphiura filiformis maintained in laboratory mesocosms. However, if increased organic input resulted in almost complete oxygen depletion, mortality of individuals was likely to occur (see de-oxygenation pressure). Mcleod et al. (2008) investigated the recovery of soft sediment benthic invertebrate community following removal of high levels of organic enrichment from fish farming in Tasmania. The authors observed that Amphiura species were associated with areas least impacted by organic enrichment. Birchenough & Frid (2009) analysed the succession of the macrobenthic community in the three years following cessation of sewage sludge disposal of the Northumberland coast, UK after 18 years of dumping. The authors reported a continued localized increase of individuals and species in the disposal area that was followed by a decline in the two sites close to the disposal site (less than 1 km). The control stations did not show this fluctuation in species abundance other than what expected because of seasonal variations. Particularly relevant was the increase in abundance of the bivalve Thyasira flexuosa. Other studies have also identified elevated Thysira flexuosa inhabiting polluted or semi-polluted areas mainly in fine sediments with high organic content (Pearson & Rosenberg, 1978; López-Jamar et al., 1987; Parra, 2002, cited in Birchenough & Frid, 2009). Similarly, Abra nitida occurs in organically enriched areas such as sediments beneath fish farms (Kutti et al., 2008). Borja et al. (2000) and Gittenberger & Van Loon (2011) both assigned Amphiura filiformis to their Ecological Group II ‘species indifferent to enrichment, always present in low densities with non-significant variations with time (from initial state, to slight unbalance)’; Abra nitida and Thyasira flexuosa were assigned to Ecological Group III ‘species tolerant to excess organic matter enrichment); Kurtiella bidentata (referred to as Mysella bidnetata), Pronucula spp. and Myrtea spinifera were characterized as AMBI Group I – ‘species very sensitive to organic enrichment and present under unpolluted conditions (initial state)’. Heteromastus filiformis was considered in both cases as an opportunistic species, tolerant to excess organic matter enrichment, although assigned to different levels (III by Borja et al., 2000, and IV by Gittenberger & Van Loon, 2011). Sensitivity assessment: No direct evidence of the characterizing species’ specific tolerances to organic enrichment was found. Typically, an increasing gradient of organic enrichment results in a decline in the suspension feeding fauna and an increase in the number of deposit feeders, in particular polychaete worms (Pearson & Rosenberg, 1978), which could result in significant change in the community composition of sedimentary habitats. Forrest et al. (2009) identified that the recovery of muddy sediments beneath fish farms from enrichment can be highly variable and may be many years at poorly flushed sites, such as those where these biotopes tend to occur. In summary, some mortality of the characterizing species of these biotopes is likely to occur, either as a direct result of a deposit of 100 gC/m2 over the period of one year, or indirect result of hypoxia. Resistance is therefore assessed as Low (loss of 25-75%), but with low confidence. Resilience is likely to be Medium, so that the biotopes are assessed as Medium sensitivity to organic enrichment at the pressure benchmark level. | LowHelp | MediumHelp | MediumHelp |
Physical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Physical loss (to land or freshwater habitat) [Show more]Physical loss (to land or freshwater habitat)Benchmark. A permanent loss of existing saline habitat within the site. Further detail EvidenceAll marine habitats and benthic species are considered to have a resistance of None to this pressure and to be unable to recover from a permanent loss of habitat (Resilience is Very Low). Sensitivity within the direct spatial footprint of this pressure is therefore High. Although no specific evidence is described, confidence in this assessment is ‘High’, due to the incontrovertible nature of this pressure. | NoneHelp | Very LowHelp | HighHelp |
Physical change (to another seabed type) [Show more]Physical change (to another seabed type)Benchmark. Permanent change from sedimentary or soft rock substrata to hard rock or artificial substrata or vice-versa. Further detail EvidenceIf the sediment that characterizes the biotopes was replaced with rock substrata, this would represent a fundamental change to the physical character of the biotopes. The characterizing species would no longer be supported and the biotopes would be lost and/or reclassified. Sensitivity assessment: Resistance to the pressure is considered None, and resilience Very Low, given the permanent nature of this pressure. Sensitivity has been assessed as High. Although no specific evidence is described, confidence in this assessment is ‘High’, due to the incontrovertible nature of this pressure. | NoneHelp | Very LowHelp | HighHelp |
Physical change (to another sediment type) [Show more]Physical change (to another sediment type)Benchmark. Permanent change in one Folk class (based on UK SeaMap simplified classification). Further detail EvidenceRecords indicate that the biotopes occur in sandy muds (Connor et al., 2004). The characterizing species within these biotopes have wide ranges of sediment preferences. For example, Amphiura filiformis has been recorded in silty mud to mixed sediment (with stones and shells) (Tillin & Tyler-Walters, 2014); Kurtiella bidentata lives in muddy sand or fine gravel (Carter, 2008); Abra spp. prefer mud, muddy gravel, muddy sand, sandy mud (Budd, 2007); and Thyasira spp. prefer mud, muddy sand, sandy mud (Jackson, 2007). Sensitivity assessment: A change in Folk class from mud and sandy mud to sand or muddy sand (based on the Long, 2006 simplification) would probably not affect the characterizing species which appear to have habitats preferences that would fall within this range. However, this would probably represent a fundamental change in the character of the biotopes, and a change in the abundance of the characteristic species, resulting in the loss and/or re-classification of the biotopes. Resistance is therefore assessed as None and resilience as Very Low and the biotopes are considered to have High sensitivity to a change in seabed type by one Folk class. | LowHelp | Very LowHelp | HighHelp |
Habitat structure changes - removal of substratum (extraction) [Show more]Habitat structure changes - removal of substratum (extraction)Benchmark. The extraction of substratum to 30 cm (where substratum includes sediments and soft rock but excludes hard bedrock). Further detail EvidenceSedimentary communities are likely to be highly intolerant of substratum removal, which will lead to partial or complete defaunation, expose underlying sediment which may be anoxic and/or of a different character and lead to changes in the topography of the area (Dernie et al., 2003). Any remaining species, given their new position at the sediment/water interface, may be exposed to unsuitable conditions. Newell et al. (1998) stated that removal of 0.5 m depth of sediment was likely to eliminate benthos from the affected area. Some epifaunal and swimming species may be able to avoid this pressure. Removal of 30 cm of sediment is likely to remove species that occur at the surface and within the upper layers of sediment, such as the characterizing species of this biotope. For example, Abra spp. are shallow burrowers and have fragile shells (Tebble, 1976). Thyasira species are found 2-8 cm below the sediment surface (Dando & Southward, 1986). Heteromastus filiformis was reported to occupy the top 15 cm of muddy sands and its limited mobility was considered to contribute to its vulnerability to dredging and to deposition of sediment mobilised by the dredging process by Shaffer (1983). Although no specific burial depths are provided for the remaining characterizing species, these are small and need to maintain contact with the surface of the sediment layer for feeding and respiration, suggesting species are unlikely to escape extraction of substratum to 30 cm. These environmental positions, together with shell fragility, are likely to render the species vulnerable to this pressure. Furthermore, dredging operations were shown to affect large infaunal and epifaunal species, decrease sessile polychaete abundance, and reduce the numbers of burrowing heart urchins (Eleftheriou & Robertson, 1992). Sensitivity assessment: Extraction of 30 cm of sediment will remove the characterizing biological component of the biotopes so resistance is assessed as None. Newell et al. (1998) indicate that local hydrodynamics (currents and wave action) and sediment characteristics (mobility and supply) strongly influence the recovery of soft sediment habitats. The biotopes occur in low energy environments, so resilience is therefore judged as Medium (see resilience section). Sensitivity has been assessed as Medium. | NoneHelp | MediumHelp | MediumHelp |
Abrasion / disturbance of the surface of the substratum or seabed [Show more]Abrasion / disturbance of the surface of the substratum or seabedBenchmark. Damage to surface features (e.g. species and physical structures within the habitat). Further detail EvidenceThe characterizing species of the biotopes are infaunal and hence have some protection against surface disturbance. However, bivalves and other species require contact with the surface for respiration and feeding, so siphons and delicate feeding structures may be damaged or withdraw because of surface disturbance, resulting in loss of feeding opportunities and compromised growth. By extending their fragile arms from the sediment to feed, characterizing species Amphiura filiformis become vulnerable to damage by abrasion. Brittlestars can resist considerable damage to arms and even the disk without suffering mortality and are capable of arm and even some disk regeneration (Sköld, 1998). Ramsay et al. (1998) suggested that Amphiura spp. may be less susceptible to beam trawl damage than other species like echinoids or tube dwelling amphipods and polychaetes. For example, Bergman & Hup (1992) found that beam trawling in the North Sea had no significant direct effect on small brittlestars. Holtmann et al. (1996) reported a decrease in the abundance of the fragile burrowing heart urchins and the brittlestar Amphiura filiformis in areas of the southern North Sea between 1990 and 1995. These trends suggest that fishing activity may have been the main cause of these changes. However, Bradshaw et al. (2002) noted that the brittlestars Amphiura filiformis had increased in abundance in a long-term study of the effects of scallop dredging in the Irish Sea. Abra spp. are shallow burrowers with a fragile shell (Tebble, 1976), and have been considered amongst the list of bivalve species most vulnerable to trawling (Bergmann & Van Santbrink, 2000) who reported between <0.5% and 18% mortality of Abra alba due to trawling in the southern North Sea. However, the small size of Abra spp. relative to meshes of commercial trawls may ensure survival of at least a moderate proportion of disturbed individuals which pass through (Rees & Dare, 1993). This is likely to be the case for small infaunal bivalve Kurtiella bidentata. Thyasira spp., are small bivalves with thin fragile shells likely to be damaged and result in mortality within the population depending on the force (Jackson, 2007). Sparks-McConkey & Watling (2001) found that trawler disturbance resulted in a decline of Thyasira flexuosa in Penobscot Bay, Maine. Heteromastus filiformis occupied the top 15 cm of muddy sands and its limited mobility was considered to contribute to its vulnerability to dredging and to deposition of sediment mobilised by the dredging process by Shaffer (1983). Rumohr & Kujawski (2000) compared qualitative historical benthos data (1902–1912) with recent data (1986) to find long-term trends in epifauna species composition in the southern North Sea that may be attributed to fishery-induced changes. In general, the frequency of occurrence of bivalve species declined, whereas scavenger and predator species (crustaceans, gastropods, and sea stars) were observed more frequently in 1986. The authors suggested that these shifts could be attributed not only to the physical fishery impact but also to the additional potential food for scavenging and predator species provided by the large amounts of discards and moribund benthos. The brittlestar Amphiura filiformis occurred in 1986 on only 5% of the stations while it was present in most of the historical stations. Also, virtually all bivalve species originally present had decreased drastically, including Nucula tenuis (also less the 5% of the sites by 1986). Despite the problems with the historical data set, the comparison presented was considered the best illustration achievable of the changes in the benthos from a near-pristine situation to the present conditions after long-term disturbance. In a meta-analysis of the impacts of different fishing activities on the benthic biota of different habitats, muddy sands were found to be vulnerable to the impacts of fishing activities, with recovery times predicted to take years (Kaiser et al., 2006). The long recovery time for muddy sands is due to the fact that these habitats are mediated by a combination of physical, chemical and biological processes (compared to sand habitats which are dominated by physical processes and recovery time takes days-months). Furthermore, abrasion events are likely to cause turbulent re-suspension of surface sediments. When used over fine muddy sediments, trawls are often fitted with shoes designed to prevent the boards digging too far into the sediment (M.J. Kaiser, pers. obs., cited in Jennings & Kaiser, 1998). The effects may persist for variable lengths of time depending on tidal strength and currents and may result in a loss of biological organization and reduce species richness (Hall, 1994; Bergman & Van Santbrink, 2000; Reiss et al., 2009) (see change in suspended solids and smothering pressures). The effects of trawling on infauna are greater in areas with low levels of natural disturbance compared to areas of high natural disturbance (e.g. Hiddink et al., 2006), and its cumulative impacts can lead to profound changes in benthic community composition, with far reaching implication for marine food webs (Hinz et al., 2009). Sensitivity assessment: Although burrowing life habits may provide some protection from damage by abrasion at the surface, a proportion of the population is likely to be damaged or removed. Significant impacts in population density would be expected if such physical disturbance were repeated at regular intervals. Furthermore, the nature of the soft sediment where the biotopes occur means that objects causing abrasion, such as fishing gears (including pots and creels) are likely to penetrate the surface and cause further damage to the characterizing species. Resistance is therefore assessed as Low and resilience as Medium, so sensitivity is assessed as Medium. | LowHelp | MediumHelp | MediumHelp |
Penetration or disturbance of the substratum subsurface [Show more]Penetration or disturbance of the substratum subsurfaceBenchmark. Damage to sub-surface features (e.g. species and physical structures within the habitat). Further detail EvidenceActivities that disturb the surface and penetrate below the surface would remove /damage infaunal species such as the characterizing species within the direct area of impact. The footprint of the impact will depend on the type of gear used (Hall et al., 2008). The biotopes occur in muddy sands (Connor et al., 2004), so penetrative activities (e.g. anchoring, scallop or suction dredging) and damage to the seabed’s sub-surface is likely to remove and/or damage the infaunal community, including the characterizing species, given that bottom fishing gears penetrate deeper into softer sediments (Bergman & Van Santbrink, 2000). Direct mortality (percentage of initial density) of Amphiura species from a single pass of a beam trawl was estimated from experimental studies on sandy and silty grounds as 9%, 20-65% for bivalves and 5-40% for gastropods, starfish, small-medium sized crustaceans and annelid worms, including Kurtiella bidentata (studied as Mysella bidentata) as 4%. Some mortality was not caused directly by the passage of the trawl, but instead by disturbance, exposure and subsequent predation (Bergman & Van Santbrink, 2000). Ball et al. (2000b) reported on the short-term effects of fishing on benthos from a mud patch in the north western part of the Irish Sea investigated in 1994–1996 by means of samples taken both before and shortly after (ca 24 hr) fishing activity. Kurtiella bidentata (studied as Mysella bidentata) was one of the species that was common at the inshore site and for which estimates of mortality were calculated and was uncommon or totally absent on the offshore fishing ground. Direct mortality from passage of an otter trawl was estimated as 70%. The delicate shells of Abra spp. are vulnerable to physical damage (e.g. by otterboards), but its small size relative to meshes of commercial trawls may ensure survival of at least a moderate proportion of disturbed individuals which pass through (Rees & Dare, 1993). Equally, Thyasira spp. also have delicate shells and are likely to be vulnerable to physical disturbance. Heteromastus filiformis was reported to occupy the top 15 cm of muddy sands and its limited mobility was considered to contribute to its vulnerability to dredging and to deposition of sediment mobilised by the dredging process by Shaffer (1983). Furthermore, penetrative events caused by a passing fishing gear are also likely to have marked impacts on the substratum and cause turbulent re-suspension of surface sediments (see abrasion pressure). When used over fine muddy sediments, trawls are often fitted with shoes designed to prevent the boards digging too far into the sediment (M.J. Kaiser, pers. obs., cited in Jennings & Kaiser, 1998). Trawling can create suspended sediment plumes up to 10 m above the bottom (Churchill, 1989 cited in Clarke & Wilber, 2000). The effects may persist for variable lengths of time depending on tidal strength and currents and may result in a loss of biological organization and reduce species richness (Hall, 1994; Bergman & Van Santbrink, 2000; Reiss et al., 2009) (see change in suspended solids and smothering pressures). A meta-analysis of over 100 experimental fishing impact studies showed that beam trawling, scallop dredging and otter trawling all had significant short-term impacts in muddy sand habitats, with most severe effect on suspension feeders (Kaiser et al., 2006). Jennings et al. (2001) found that trawling in the muddy sand region led to significant decreases in infaunal biomass and production in the North Sea, with the abundance of larger individuals depleted more than smaller ones. Sensitivity assessment: A large proportion of the characterizing species in these biotopes is likely to be lost or severely damaged, depending on the scale of the activity (see abrasion pressure). Therefore, a resistance of Low is suggested. Muddy sand habitats have been reported as having the longest recovery times, whilst mud habitats had an ‘intermediate’ recovery time (compared to clean sand communities which had the most rapid recovery rate) (Dernie et al., 2003). Resilience is probably Medium, and therefore the biotopes’ sensitivity to this pressure is likely to be Medium. | LowHelp | MediumHelp | MediumHelp |
Changes in suspended solids (water clarity) [Show more]Changes in suspended solids (water clarity)Benchmark. A change in one rank on the WFD (Water Framework Directive) scale e.g. from clear to intermediate for one year. Further detail EvidenceThe biotopes are found in weak and very weak tidal streams (Connor et al., 2004). Clogging of feeding apparatus by suspended sediment is likely to be a major consideration for the characterizing species of the biotopes, which include a number of suspension feeders, such as brittlestar Amphiura filiformis, and bivalves Kurtiella bidentata, Abra spp., Pronucula nitida and Thyasira spp.. For example, according to Widdows et al. (1979) growth of filter-feeding bivalves may be impaired at suspended particulate matter (SPM) concentrations >250 mg/l. For instance, the abundance of Abra alba declined over two years within 1 km of an outfall pipe discharging fine-grained mineral waste from the china/clay industry at a rate of 450,000 tons per year to Mevagissey Bay, Cornwall. However, it was argued that persistent sediment instability was the more significant source of stress to the predominantly deposit-feeding community than the suspended sediment concentration (Probert, 1981). Amphiura filiformi, Kurtiella bidentata and Abra spp. are able to switch between feeding methods (Hill & Wilson, 2008; Carter, 2008; Budd, 2007) and are likely to change to deposit feeding in stagnant waters or areas of very low water flow (Ockelmann & Muus, 1978). The characterizing polychaetes of the biotopes are thought to be predators or deposit feeders. For most benthic deposit feeders, food is suggested to be a limiting factor for body and gonad growth, at least between events of sedimentation of fresh organic matter (Hargrave, 1980; Tenore, 1988). Consequently, increased organic matter in suspension that is deposited may become incorporated into sediments via bioturbation and may enhance food supply. A decrease in the suspended sediment and hence siltation may reduce the flux of particulate material to the seabed. Since this includes organic matter the supply of food to the biotopes would probably also be reduced. While regenerating arms, the amount of food the brittlestars can feed on is decreased, meaning there is less energy to allocate to arm regeneration. If there is a change in the amount and quality of food available because of change in suspended solids in the biotopes, then this can have aggravated effects of the growth and development of brittlestars (Lawrence, 2010). Where a change in suspended solids results in increased turbidity and change of light, the community is unlikely to be directly affected. The community is also unlikely to be directly affected by increased light penetration of the water column caused by a decrease in turbidity. Greater light penetration of the water column may improve primary production by phytoplankton in the water column and contribute to secondary productivity via the production of detritus from which the community may benefit. Sensitivity assessment: An increase in the suspended matter settling out from the water column to the substratum may increase food availability. On the other hand, decreased siltation is unlikely to affect the mainly deposit feeding community that occur in the biotopes. Resistance of the biotopes is likely to be High, but with low confidence as no direct evidence was found. Resistance is likely to be High (by default) and the biotopes are, therefore, assessed as Not Sensitive to a change in suspended solids at the pressure benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Smothering and siltation rate changes (light) [Show more]Smothering and siltation rate changes (light)Benchmark. ‘Light’ deposition of up to 5 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceThe biotopes are characterized by burrowing species that are likely to be able to burrow upwards and therefore unlikely to be adversely affected by smothering of 5 cm sediment. Last et al. (2011) buried Ophiura ophiura individuals under three different depths of sediment; shallow (2 cm), medium (5 cm) and deep (7 cm). The results indicated that Ophiura ophiura is highly tolerant of short-term (32 days) burial events, with less than 10% mortality of all buried specimens. This is largely a reflection of the ability of the species to re-emerge from all depths across all sediment fractions tested. Survival of specimens that remained buried was low, with 100% mortality of individuals that remained buried after 32 days. The experiments utilized three different fractions of kiln dried, commercially obtained marine sediment: coarse (1.2-2.0mm diameter), medium fine (0.25-0.95mm diameter) and fine (0.1-0.25mm diameter). Trannum et al. (2010) investigated how sedimentation from water-bases drill cuttings could affect benthic communities, in comparison with natural sediment deposition. The authors concluded there was no effect of adding natural test sediment up to 2.4 cm but a significant reduction in number of taxa, abundance, biomass and diversity of fauna with increasing layer of thickness of drill cuttings (3-24 mm), suggesting other mechanisms affecting the fauna other than sedimentation, possibly lower contents of nutrients, toxicity and oxygen depletion. Amphiura filiformis was amongst the species to be absent from treatments under 6, 12 and 24 mm of artificial sediment, possibly due to its surface deposit feeding habits. Characterizing suspension feeders may not persist in areas of excessive sedimentation. Material in suspension can affect the efficiency of filter and suspension feeding (Sherk & Cronin, 1970; Morton, 1976). Effects can include abrasion and clogging of gills, impaired respiration, clogging of filter mechanisms, and reduced feeding and pumping rates. Hinchey et al. (2006) investigated the responses of estuarine benthic invertebrates to sediment burial and concluded that species-specific response to burial varied as a function of motility living position, and physiological tolerance of anoxic conditions while buried. Although the characterizing species were not included in the study, increased overburden stress did not significantly decrease survival and growth of the juvenile bivalve studied, Macoma balthica, but significantly caused decline juvenile Streblospio benedicti. The depth of sediment deposited varied between 0-24.6 cm and 0-8.4 cm, respectively. Furthermore, a study of the ecological effects of dumping dredged sediments by Essink (1999) reported that resistance of mobile macrobenthos varied greatly with species. For polychaetes, the author reported tolerances of up to 50 cm of mud for species such as Nepthys and Nereis, and up to 80 cm of sand. Bijkerk (1988, results cited from Essink, 1999) indicated that the maximal overburden through which small bivalves could migrate was 20 cm in sand for Donax and approximately 40 cm in mud for Tellina sp. and approximately 50 cm in sand. Powilleit et al. (2009) studied responses to smothering for three bivalves; Arctica islandica, Macoma balthica and Mya arenaria. These successfully burrowed to the surface of a 32 – 41 cm deposited sediment layer of till or sand/till mixture and restored contact with the overlying water. These high escape potentials could partly be explained by the heterogeneous texture of the till and sand/till mixture with ‘voids’. In comparison to a thick coverage, thin covering layers (i.e. 15 - 16 cm and 20 cm) increased the chance of the organisms to reach the sediment surface after burial. This suggests that characterizing species such as Kurtiella bidentata, Abra spp., Thyasira spp. and Pronucula tenuis are likely to be able to reborrow through similar overburdens, although sudden smothering with 5 cm of sediment would temporarily halt feeding and respiration, compromising growth and reproduction owing to energetic expenditure. Furthermore, Thyasira flexuosa have highly extensible feet (Dando & Southward, 1986) allowing them to construct channels within the sediment and to burrow to 8 cm depth. Being adapted for burrowing means these species are likely to resist additional fine sediment. However, it should be remembered that smothering by impermeable or viscous materials are likely to have some effect upon the animals, e.g. by causing deoxygenation. Sensitivity assessment: Beyond re-establishing burrow openings or moving up through the sediment, there is evidence of synergistic effects on burrowing activity of marine benthos and mortality with changes in time of burial, sediment depth, sediment type and temperature (Maurer et al., 1986). However, the biotopes are likely to resist smothering at the benchmark level since the majority of associated fauna are burrowing infauna. Resistance is therefore assessed as High, and resilience is also High (by default) so the biotopes are considered Not Sensitive to a ‘light’ deposition of up to 5 cm of fine material added to the seabed in a single, discrete event. | HighHelp | HighHelp | Not sensitiveHelp |
Smothering and siltation rate changes (heavy) [Show more]Smothering and siltation rate changes (heavy)Benchmark. ‘Heavy’ deposition of up to 30 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceThe biotopes are characterized by burrowing species that are likely to be able to burrow upwards. Last et al. (2011) buried Ophiura ophiura individuals under three different depths of sediment; shallow (2 cm), medium (5 cm) and deep (7 cm). The results indicated that Ophiura ophiura is highly tolerant of short-term (32 days) burial events, with less than 10% mortality of all buried specimens. This is largely a reflection of the ability of the species to re-emerge from all depths across all sediment fractions tested. Survival of specimens that remained buried was low, with 100% mortality of individuals that remained buried after 32 days. The experiments utilized three different fractions of kiln dried, commercially obtained marine sediment: coarse (1.2-2.0mm diameter), medium fine (0.25-0.95mm diameter) and fine (0.1-0.25mm diameter). Trannum et al. (2010) investigated how sedimentation from water-bases drill cuttings could affect benthic communities, in comparison with natural sediment deposition. The authors concluded there was no effect of adding natural test sediment up to 2.4 cm but a significant reduction in number of taxa, abundance, biomass and diversity of fauna with increasing layer of thickness of drill cuttings (3-24 mm), suggesting other mechanisms affecting the fauna other than sedimentation, possibly lower contents of nutrients, toxicity and oxygen depletion. Amphiura filiformis was amongst the species to be absent from treatments under 6, 12 and 24 mm of artificial sediment, possibly due to its surface deposit feeding habits. Characterizing suspension feeders may not persist in areas of excessive sedimentation. Material in suspension can affect the efficiency of filter and suspension feeding (Sherk & Cronin, 1970; Morton, 1976). Effects can include abrasion and clogging of gills, impaired respiration, clogging of filter mechanisms, and reduced feeding and pumping rates. Hinchey et al. (2006) investigated the responses of estuarine benthic invertebrates to sediment burial and concluded that species-specific response to burial varied as a function of motility living position, and physiological tolerance of anoxic conditions while buried. Although the characterizing species were not included in the study, increased overburden stress did not significantly decrease survival and growth of the juvenile bivalve studied, Macoma balthica, but significantly caused decline juvenile Streblospio benedicti. The depth of sediment deposited varied between 0-24.6 cm and 0-8.4 cm, respectively. Furthermore, a study of the ecological effects of dumping dredged sediments by Essink (1999) reported that resistance of mobile macrobenthos varied greatly with species. For polychaetes, the author reported tolerances of up to 50 cm of mud for species such as Nepthys and Nereis, and up to 80 cm of sand. Bijkerk (1988, results cited from Essink, 1999) indicated that the maximal overburden through which small bivalves could migrate was 20 cm in sand for Donax and approximately 40 cm in mud for Tellina sp. and approximately 50 cm in sand. Powilleit et al. (2009) studied responses to smothering for three bivalves; Arctica islandica, Macoma balthica and Mya arenaria. These successfully burrowed to the surface of a 32 – 41 cm deposited sediment layer of till or sand/till mixture and restored contact with the overlying water. These high escape potentials could partly be explained by the heterogeneous texture of the till and sand/till mixture with ‘voids’. In comparison to a thick coverage, thin covering layers (i.e. 15 - 16 cm and 20 cm) increased the chance of the organisms to reach the sediment surface after burial. This suggests that characterizing species such as Kurtiella bidentata, Abra spp., Thyasira spp. and Pronucula tenuis are likely to be able to reborrow through similar overburdens, although sudden smothering with 5 cm of sediment would temporarily halt feeding and respiration, compromising growth and reproduction owing to energetic expenditure. Furthermore, Thyasira flexuosa have highly extensible feet (Dando & Southward, 1986) allowing them to construct channels within the sediment and to burrow to 8 cm depth. Being adapted for burrowing means these species are likely to resist additional fine sediment. However, it should be remembered that smothering by impermeable or viscous materials are likely to have some effect upon the animals, e.g. by causing deoxygenation. Sensitivity assessment: Beyond re-establishing burrow openings or moving up through the sediment, there is evidence of synergistic effects on burrowing activity of marine benthos and mortality with changes in time of burial, sediment depth, sediment type and temperature (Maurer et al., 1986). Bivalve and polychaete species have been reported to migrate through depositions of sediment greater that the benchmark (30 cm of fine material added to the seabed in a single discrete event) (Bijkerk, 1988; Powilleit et al., 2009; Maurer et al., 1982). However, it is not clear whether the characterizing species are likely to be able to migrate through a maximum thickness of fine sediment because muds tend to be more cohesive and compacted than sand. Some mortality of the characterizing species is likely to occur. Resistance is therefore assessed as Low (25-75% loss), but with low confidence. Resilience as Medium and the biotopes are considered to have Medium sensitivity to a ‘heavy’ deposition of up to 30 cm of fine material in a single discrete event. | LowHelp | MediumHelp | MediumHelp |
Litter [Show more]LitterBenchmark. The introduction of man-made objects able to cause physical harm (surface, water column, seafloor or strandline). Further detail EvidenceNot assessed. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Electromagnetic changes [Show more]Electromagnetic changesBenchmark. A local electric field of 1 V/m or a local magnetic field of 10 µT. Further detail EvidenceNo Evidence is available on which to assess this pressure. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Underwater noise changes [Show more]Underwater noise changesBenchmark. MSFD indicator levels (SEL or peak SPL) exceeded for 20% of days in a calendar year. Further detail EvidenceSpecies in the biotopes may respond to vibrations from predators or excavation by retracting their palps or by burrowing deeper into the sediment. However, the characterizing species are unlikely to be affected by noise pollution and so the biotopes are assessed as Not Sensitive. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Introduction of light or shading [Show more]Introduction of light or shadingBenchmark. A change in incident light via anthropogenic means. Further detail EvidenceThe biotopes are circalittoral (Connor et al., 2004) and therefore, not directly dependent on sunlight. Sensitivity assessment: The biotopes are considered to have High resistance and, by default, High resilience and therefore is Not Sensitive to this pressure. | Not relevant (NR)Help | Not relevant (NR)Help | Not sensitiveHelp |
Barrier to species movement [Show more]Barrier to species movementBenchmark. A permanent or temporary barrier to species movement over ≥50% of water body width or a 10% change in tidal excursion. Further detail EvidenceNot Relevant to biotopes restricted to open waters. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Death or injury by collision [Show more]Death or injury by collisionBenchmark. Injury or mortality from collisions of biota with both static or moving structures due to 0.1% of tidal volume on an average tide, passing through an artificial structure. Further detail EvidenceNot Relevant to seabed habitats. NB. Collision by grounding vessels is addressed under surface abrasion | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Visual disturbance [Show more]Visual disturbanceBenchmark. The daily duration of transient visual cues exceeds 10% of the period of site occupancy by the feature. Further detail EvidenceThe characterizing species of the biotopes live infaunally, so are likely to have poor or no visual perception and unlikely to be affected by visual disturbance such as shading. For example, movement of a hand near brittlestar Ophiothrix fragilis elicits no escape response (Sköld, 1998). | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Biological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Genetic modification & translocation of indigenous species [Show more]Genetic modification & translocation of indigenous speciesBenchmark. Translocation of indigenous species or the introduction of genetically modified or genetically different populations of indigenous species that may result in changes in the genetic structure of local populations, hybridization, or change in community structure. Further detail EvidenceThe important characterizing species in the biotopes are not cultivated or likely to be translocated. This pressure is therefore considered Not Relevant. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Introduction or spread of invasive non-indigenous species [Show more]Introduction or spread of invasive non-indigenous speciesBenchmark. The introduction of one or more invasive non-indigenous species (INIS). Further detail EvidenceThe American slipper limpet Crepidula fornicata was introduced to the UK and Europe in the 1870s from the Atlantic coasts of North America with imports of the eastern oyster Crassostrea virginica. It was recorded in Liverpool in 1870 and the Essex coast in 1887-1890. It has spread through expansion and introductions along the full extent of the English Channel and into the European mainland (Blanchard, 1997, 2009; Bohn et al., 2012, 2013a, 2013b, 2015; De Montaudouin et al., 2018; Helmer et al., 2019; Hinz et al., 2011; McNeill et al., 2010; Powell-Jennings & Calloway, 2018; Preston et al., 2020; Stiger-Pouvreau & Thouzeau, 2015). Crepidula fornicata is recorded from shallow, sheltered bays, lagoons and estuaries or the sheltered sides of islands, in variable salinity (18 to 40) although it prefers ca 30 (Tillin et al., 2020). Larvae require hard substrata for settlement. It prefers muddy gravelly, shell-rich, substrata that include gravel, or shells of other Crepidula, or other species e.g., oysters, and mussels. It is highly gregarious and seeks out adult shells for settlement, forming characteristic ‘stacks’ of adults. But it also recorded in a wide variety of habitats including clean sands, artificial substrata, Sabellaria alveolata reefs and areas subject to moderately strong tidal streams (Blanchard, 1997, 2009; Bohn et al., 2012, 2013a, 2013b, 2015; De Montaudouin et al., 2018; Hinz et al., 2011; Powell-Jennings & Calloway, 2018; Preston et al., 2020; Stiger-Pouvreau & Thouzeau, 2015; Tillin et al., 2020). High densities of Crepidula fornicata cause ecological impacts on sedimentary habitats. The species can form dense carpets that can smother the seabed in shallow bays, changing and modifying the habitat structure. At high densities, the species physically smothers the sediment, and the resultant build-up of silt, pseudofaeces, and faeces is deposited and trapped within the bed (Tillin et al., 2020, Fitzgerald, 2007, Blanchard, 2009, Stiger-Pouvreau & Thouzeau, 2015). The biodeposition rates of Crepidula are extremely high and once deposited, form an anoxic mud, making the environment suitable for other species, including most infauna (Stiger-Pouvreau & Thouzeau, 2015, Blanchard, 2009). For example, in fine sands, the community is replaced by a reef of slipper limpets, that provide hard substrata for sessile suspension-feeders (e.g., sea squirts, tube worms and fixed shellfish), while mobile carnivorous microfauna occupy species between or within shells, resulting in a homogeneous Crepidula dominated habitat (Blanchard, 2009). Blanchard (2009) suggested the transition occurred and became irreversible at 50% cover of the limpet. De Montaudouin et al. (2018) suggested that homogenization occurred above a threshold of 20-50 Crepidula /m2. Impacts on the structure of benthic communities will depend on the type of habitat that Crepidula colonizes. De Montaudouin & Sauriau (1999) reported that in muddy sediment dominated by deposit-feeders, species richness, abundance and biomass increased in the presence of high densities of Crepidula (ca 562 to 4772 ind./m2), in the Bay of Marennes-Oléron, presumably because the Crepidula bed provided hard substrata in an otherwise sedimentary habitat. In medium sands, Crepidula density was moderate (330-1300 ind./m2) but there was no significant difference between communities in the presence of Crepidula. Intertidal coarse sediment was less suitable for Crepidula with only moderate or low abundances (11 ind./m2) and its presence did not affect the abundance or diversity of macrofauna. However, there was a higher abundance of suspension–feeders and mobile Crustacea in the absence of Crepidula (De Montaudouin & Sauriau, 1999). The presence of Crepidula as an ecosystem engineer has created a range of new niche habitats, reducing biodiversity as it modifies habitats (Fitzgerald, 2007). De Montaudouin et al. (1999) concluded that Crepidula did not influence macroinvertebrate diversity or density significantly under experimental conditions, on fine sands in Arcachon Bay, France. De Montaudouin et al. (2018) noted that the slipper limpet reef increased the species diversity in the bed, but homogenised diversity compared to areas where the limpets were absent. In the Milford Haven Waterway (MHW), the highest densities of Crepidula were found in areas of sediment with hard substrata, e.g., mixed fine sediment with shell or gravel or both (grain sizes 16-256 mm) but, while Crepidula density increased as gravel cover increased in the subtidal, the reverse was found in the intertidal (Bohn et al., 2015). Bohn et al. (2015) suggested that high densities of Crepidula in high-energy environments were possible in the subtidal but not the intertidal, suggesting the availability of this substratum type is beneficial for its establishment. Hinz et al. (2011) reported a substantial increase in the occurrence of Crepidula off the Isle of Wight, between 1958 and 2006, at a depth of ca 60 m, on hard substrata (gravel, cobbles, and boulders), swept by strong tidal streams. Presumably, Crepidula is more tolerant of tidal flow than the oscillatory flow caused by wave action which may be less suitable (Tillin et al., 2020). The availability of hard substrata (e.g., gravel) may only restrict initial colonization as higher densities of Crepidula function as substrata for subsequent colonization (Thieltges et al., 2004; Blanchard, 2009). However, Bohn et al. (2015) noted that Crepidula occurred at low density or was absent in areas of homogenous fine sediment and areas dominated by boulders. Bohn et al. (2015) suggested that wave action (exposure) probably prevented the establishment of large numbers of Crepidula in high-energy areas. Blanchard (2009) noted that sandy areas in the Bay of Saint-Mont Michel were not colonized by Crepidula because of surface sand mobility. Thieltges et al. (2003) also noted that storm events removed some clumps of mussels and presumably Crepidula onto tidal flats where they disappeared, which caused their abundance to fluctuate. Similarly, Crepidula was absent from sandy substrata in Swansea Bay but was most abundant in the shelter of the breakwater at the Swansea east site (Powell-Jennings & Calloway, 2018). Sensitivity assessment. The sediments characterizing this biotope are likely to be too mobile and unsuitable for most of the invasive non-indigenous species currently recorded in the UK. The above evidence suggests that this biotope is unsuitable for the colonization of Crepidula fornicata due to a lack of gravel, shell, or any other hard substrata used for larvae settlement (Tillin et al., 2020). This habitat is moderately exposed (JNCC, 2022), in which storms may mobilise the sediment, and which may mitigate or prevent colonization by Crepidula at high densities. Hence, resistance is assessed as 'High' and resilience as 'High' so that the biotope is assessed as 'Not sensitive'. | HighHelp | HighHelp | Not sensitiveHelp |
Introduction of microbial pathogens [Show more]Introduction of microbial pathogensBenchmark. The introduction of relevant microbial pathogens or metazoan disease vectors to an area where they are currently not present (e.g. Martelia refringens and Bonamia, Avian influenza virus, viral Haemorrhagic Septicaemia virus). Further detail EvidenceIntroduced organisms (especially parasites or pathogens) are a potential threat in all coastal ecosystems. Several examples are known of echinoderm populations that have been massively reduced by sudden outbreaks of epidemic disease. Cases include the mass mortality of the sea urchin Diadema antillarum throughout the Caribbean as a result of infection by a water-borne pathogen (Lessios, 1988), and the decimation of urchin populations in the North Atlantic by parasitic amoebae and nematodes (Hagen, 1997). Brittlestars have symbiotic sub-cuticular bacteria. The host-bacteria association can be perturbed by acute stress and changes in bacterial loading may be used as an indicator of sub-lethal stress (Newton & McKenzie, 1995). More than 20 viruses have been described for marine bivalves (Sinderman, 1990). Bacterial diseases are more significant in the larval stages and protozoans are the most common cause of epizootic outbreaks that may result in mass mortalities of bivalve populations. Parasitic worms, trematodes, cestodes and nematodes can reduce growth and fecundity within bivalves and may in some instances cause death (Dame, 1996). A viral infection of the mutualist bacterium living on the gills of Thyasira gouldii was suggested as the reason for a major decline in the Loch Etive population (Jackson, 2007). However, no information specifically concerning the effects of microbial pathogens and parasites on the viability of the characterizing species was found. Sensitivity assessment: No direct evidence of the biotopes being affected by the introduction of microbial pathogens was found as with which to assess this pressure. This pressure is therefore assessed as No Evidence. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Removal of target species [Show more]Removal of target speciesBenchmark. Removal of species targeted by fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidenceIt is extremely unlikely that any of the species indicative of sensitivity would be targeted for extraction. This pressure is therefore considered Not Relevant. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Removal of non-target species [Show more]Removal of non-target speciesBenchmark. Removal of features or incidental non-targeted catch (by-catch) through targeted fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidenceDirect, physical impacts are assessed through the abrasion and penetration of the seabed pressures, while this pressure considers the ecological or biological effects of by-catch. Species in these biotopes, including the characterizing species, may be damaged or directly removed by static or mobile gears that are targeting other species (see abrasion and penetration pressures). Loss of these species would alter the character of the biotope resulting in reclassification, and would alter the physical structure of the habitat resulting in the loss of the ecosystem functions such as secondary production performed by these species. Sensitivity assessment: Removal of the characterizing species would result in the biotopes being lost or reclassified. Thus, the biotopes are assessed to have a resistance of Low to this pressure and to have Medium resilience, resulting in the sensitivity being judged as Medium. | LowHelp | MediumHelp | MediumHelp |
Bibliography
Addy, J.M., Levell, D. & Hartley, J.P., 1978. Biological monitoring of sediments in the Ekofisk oilfield. In Proceedings of the conference on assessment of ecological impacts of oil spills. American Institute of Biological Sciences, Keystone, Colorado 14-17 June 1978, pp.514-539.
Aronson, R.B., 1990. Onshore-offshore patterns of human fishing activity. Palaios, 5, 88-93.
Baden, S.P., Pihl, L. & Rosenberg, R., 1990. Effects of oxygen depletion on the ecology, blood physiology and fishery of the Norway lobster Nephrops norvegicus. Marine Ecology Progress Series, 67, 141-155.
Ball, B., Munday, B. & Tuck, I., 2000b. Effects of otter trawling on the benthos and environment in muddy sediments. In: Effects of fishing on non-target species and habitats, (eds. Kaiser, M.J. & de Groot, S.J.), pp 69-82. Oxford: Blackwell Science.
Bergman, M.J.N. & Hup, M., 1992. Direct effects of beam trawling on macrofauna in a sandy sediment in the southern North Sea. ICES Journal of Marine Science, 49, 5-11. DOI https://doi.org/10.1093/icesjms/49.1.5
Bergman, M.J.N. & Van Santbrink, J.W., 2000b. Fishing mortality of populations of megafauna in sandy sediments. In The effects of fishing on non-target species and habitats (ed. M.J. Kaiser & S.J de Groot), 49-68. Oxford: Blackwell Science.
Beukema, J.J., 1979. Biomass and species richness of the macrobenthic animals living on a tidal flat area in the Dutch Wadden Sea: effects of a severe winter. Netherlands Journal of Sea Research, 13, 203-223.
Birchenough, S. N. & C. L. Frid, 2009. Macrobenthic succession following the cessation of sewage sludge disposal. Journal of Sea Research 62 (4), 258-267.
Blanchard, M., 2009. Recent expansion of the slipper limpet population (Crepidula fornicata) in the Bay of Mont-Saint-Michel (Western Channel, France). Aquatic Living Resources, 22 (1), 11-19. DOI https://doi.org/10.1051/alr/2009004
Blanchard, M., 1997. Spread of the slipper limpet Crepidula fornicata (L.1758) in Europe. Current state and consequences. Scientia Marina, 61, Supplement 9, 109-118. Available from: http://scimar.icm.csic.es/scimar/index.php/secId/6/IdArt/290/
Bohn, K., Richardson, C. & Jenkins, S., 2012. The invasive gastropod Crepidula fornicata: reproduction and recruitment in the intertidal at its northernmost range in Wales, UK, and implications for its secondary spread. Marine Biology, 159 (9), 2091-2103. DOI https://doi.org/10.1007/s00227-012-1997-3
Bohn, K., Richardson, C.A. & Jenkins, S.R., 2015. The distribution of the invasive non-native gastropod Crepidula fornicata in the Milford Haven Waterway, its northernmost population along the west coast of Britain. Helgoland Marine Research, 69 (4), 313.
Bohn, K., Richardson, C.A. & Jenkins, S.R., 2013a. Larval microhabitat associations of the non-native gastropod Crepidula fornicata and effects on recruitment success in the intertidal zone. Journal of Experimental Marine Biology and Ecology, 448, 289-297. DOI https://doi.org/10.1016/j.jembe.2013.07.020
Bohn, K., Richardson, C.A. & Jenkins, S.R., 2013b. The importance of larval supply, larval habitat selection and post-settlement mortality in determining intertidal adult abundance of the invasive gastropod Crepidula fornicata. Journal of Experimental Marine Biology and Ecology, 440, 132-140. DOI https://doi.org/10.1016/j.jembe.2012.12.008
Borja, A., Franco, J. & Perez, V., 2000. A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Marine Pollution Bulletin, 40 (12), 1100-1114.
Bradshaw, C., Veale, L.O., Hill, A.S. & Brand, A.R., 2002. The role of scallop-dredge disturbance in long-term changes in Irish Sea benthic communities: a re-analysis of an historical dataset. Journal of Sea Research, 47, 161-184. DOI https://doi.org/10.1016/S1385-1101(02)00096-5
Bryan, G.W., 1984. Pollution due to heavy metals and their compounds. In Marine Ecology: A Comprehensive, Integrated Treatise on Life in the Oceans and Coastal Waters, vol. 5. Ocean Management, part 3, (ed. O. Kinne), pp.1289-1431. New York: John Wiley & Sons.
Buchanan, J.B. & Warwick, R.M., 1974. An estimate of benthic macrofaunal production in the offshore mud of the Northumberland coast. Journal of the Marine Biological Association of the United Kingdom, 54, 197-222. DOI https://doi.org/10.1017/S0025315400022165
Buchanan, J.B., 1964. A comparative study of some of the features of the biology of Amphiura filiformis and Amphiura chiajei (Ophiuroidea) considered in relation to their distribution. Journal of the Marine Biological Association of the United Kingdom, 44, 565-576.
Buchanan, J.B., 1966. The biology of Echinocardium cordatum (Echinodermata: Spatangoidea) from different habitats. Journal of the Marine Biological Association of the United Kingdom, 46, 97-114.
Buchanan, J.B., 1967. Dispersion and demography of some infaunal echinoderm populations. Symposia of the Zoological Society of London, 20, 1-11.
Budd, G.C. 2007. Abra alba White furrow shell. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. Available from: http://www.marlin.ac.uk/species/detail/1722
Buhr, K.-J., 1981. Effects of the cold winter 1978/79 on the macrobenthos of the Lanice-association in the Weser Estuary. Veroffentlichungen des Instituts fur Meeresforschung in Bremerhaven, 19, 115-131.
Cabioch, L., Dauvin, J.C. & Gentil, F., 1978. Preliminary observations on pollution of the sea bed and disturbance of sub-littoral communities in northern Brittany by oil from the Amoco Cadiz. Marine Pollution Bulletin, 9, 303-307.
Can, E., Kevrekidis, T. & Cihangir, B., 2012. Factors affecting monthly variation in populationdensity of the capitellid polychaete Heteromastus filiformis in a hyperhaline Mediterranean coastal lagoon. Transitional Waters Bulletin, 3(3), 10-23.
Carter, M.C. 2008. Kurtiella bidentata A bivalve mollusc. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. Available from: http://www.marlin.ac.uk/species/detail/1939
Carvalho, F.P., 2011. Polonium (210 Po) and lead (210 Pb) in marine organisms and their transfer in marine food chains. Journal of Environmental Radioactivity, 102 (5), 462-472.
Caspers, H., 1981. Long-term changes in benthic fauna resulting from sewage sludge dumping in the North Sea. Water Science and Technology, 13, 461-479.
Chauvaud, L., Jean, F., Ragueneau, O. & Thouzeau, G., 2000. Long-term variation of the Bay of Brest ecosystem: benthic-pelagic coupling revisited. Marine Ecology Progress Series, 200, 35-48. DOI https://doi.org/10.3354/meps200035
Clark, R.B., 1997. Marine Pollution, 4th edition. Oxford: Carendon Press.
Clarke, D.G. & Wilber, D.H. 2000. Assessment of potential impacts of dredging operations due to sediment resuspension. DOER Technical Notes Collection (ERDCTN-DOER-E9), U.S. Army Engineer Research and Development Centre, Vicksburge, MS.
Cole, S., Codling, I.D., Parr, W. & Zabel, T., 1999. Guidelines for managing water quality impacts within UK European Marine sites. Natura 2000 report prepared for the UK Marine SACs Project. 441 pp., Swindon: Water Research Council on behalf of EN, SNH, CCW, JNCC, SAMS and EHS. [UK Marine SACs Project.]. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/water_quality.pdf
Connor, D.W., Allen, J.H., Golding, N., Howell, K.L., Lieberknecht, L.M., Northen, K.O. & Reker, J.B., 2004. The Marine Habitat Classification for Britain and Ireland. Version 04.05. ISBN 1 861 07561 8. In JNCC (2015), The Marine Habitat Classification for Britain and Ireland Version 15.03. [2019-07-24]. Joint Nature Conservation Committee, Peterborough. Available from https://mhc.jncc.gov.uk/
Connor, D.W., Dalkin, M.J., Hill, T.O., Holt, R.H.F. & Sanderson, W.G., 1997a. Marine biotope classification for Britain and Ireland. Vol. 2. Sublittoral biotopes. Joint Nature Conservation Committee, Peterborough, JNCC Report no. 230, Version 97.06., Joint Nature Conservation Committee, Peterborough, JNCC Report no. 230, Version 97.06.
Coulon, P. & Jangoux, M., 1987. Gregarine species (Apicomplexa) parasitic in the burrowing echinoid Echinocardium cordatum: occurrence and host reaction. Diseases of Aquatic Organisms, 2, 135-145.
Coyle, K.O., Konar, B., Blanchard, A., Highsmith, R.C., Carroll, J., Carroll, M., Denisenko, S.G. & Sirenko, B.I., 2007. Potential effects of temperature on the benthic infaunal community on the southeastern Bering Sea shelf: Possible impacts of climate change. Deep Sea Research Part II: Topical Studies in Oceanography, 54 (23–26), 2885-2905.
Crisp, D.J. (ed.), 1964. The effects of the severe winter of 1962-63 on marine life in Britain. Journal of Animal Ecology, 33, 165-210.
Crompton, T.R., 1997. Toxicants in the aqueous ecosystem. New York: John Wiley & Sons.
Daan, R. & Mulder, M., 1996. On the short-term and long-term impact of drilling activities in the Dutch sector of the North Sea ICES Journal of Marine Science, 53, 1036-1044.
Daan, R., Groenewould Van Het, H., Jong De, S.A. & Mulder, M., 1992. Physico-chemical and biological features of a drilling site in the North Sea, 1 year after discharges of oil-contaminated drill cuttings. Marine Ecology Progress Series, 91, 37-45.
Dahllöf, I., Blanck, H., Hall, P.O.J. & Molander, S., 1999. Long term effects of tri-n-butyl-tin on the function of a marine sediment system. Marine Ecology Progress Series, 188, 1-11.
Dame, R.F.D., 1996. Ecology of Marine Bivalves: an Ecosystem Approach. New York: CRC Press Inc. [Marine Science Series.]
Dando, P.R. & Southward, A.J., 1986. Chemoautotrophy in bivalve molluscs of the Genus Thyasira. Journal of the Marine Biological Association of the United Kingdom, 60, 915-929.
Dando, P.R. & Spiro, B., 1993. Varying nutritional dependence of the thyasirid bivalves Thyasira sarsi and Thyasira equalis on chemoautotrophic symbiotic bacteria, demonstrated by isotope ratios of tissue carbon and shell carbonate. Marine Ecology Progress Series, 92, 151-158.
Dauvin, J-C. & Gentil, F., 1989. Long-term changes in populations of subtidal bivalves (Abra alba and Abra prismatica) from the Bay of Morlaix (Western English Channel). Marine Biology, 103, 63-73.
Dauvin, J.C., 1982. Impact of Amoco Cadiz oil spill on the muddy fine sand Abra alba - Melinna palmata community from the Bay of Morlaix. Estuarine and Coastal Shelf Science, 14, 517-531.
Dauvin, J.C., 1998. The fine sand Abra alba community of the Bay of Morlaix twenty years after the Amoco Cadiz oil spill. Marine Pollution Bulletin, 36, 669-676.
Dauwe, B., Herman, P.M.J. & Heip, C.H.R., 1998. Community structure and bioturbation potential of macrofauna at four North Sea stations with contrasting food supply. Marine Ecology Progress Series, 173, 67-83.
Davies, C.E. & Moss, D., 1998. European Union Nature Information System (EUNIS) Habitat Classification. Report to European Topic Centre on Nature Conservation from the Institute of Terrestrial Ecology, Monks Wood, Cambridgeshire. [Final draft with further revisions to marine habitats.], Brussels: European Environment Agency.
Davis, J.P. & Wilson, J.G., 1983b. The population structure and ecology of Nucula turgida (Leckenby & Marshall) in Dublin Bay. Progress in Underwater Science, 8, 53-60.
De Montaudouin, X. & Sauriau, P.G., 1999. The proliferating Gastropoda Crepidula fornicata may stimulate macrozoobenthic diversity. Journal of the Marine Biological Association of the United Kingdom, 79, 1069-1077. DOI https://doi.org/10.1017/S0025315499001319
De Montaudouin, X., Andemard, C. & Labourg, P-J., 1999. Does the slipper limpet (Crepidula fornicata L.) impair oyster growth and zoobenthos diversity ? A revisited hypothesis. Journal of Experimental Marine Biology and Ecology, 235, 105-124.
De Montaudouin, X., Blanchet, H. & Hippert, B., 2018. Relationship between the invasive slipper limpet Crepidula fornicata and benthic megafauna structure and diversity, in Arcachon Bay. Journal of the Marine Biological Association of the United Kingdom, 98 (8), 2017-2028. DOI https://doi.org/10.1017/s0025315417001655
Deheyn, D.D. & Latz, M.I., 2006. Bioavailability of metals along a contamination gradient in San Diego Bay (California, USA). Chemosphere, 63 (5), 818-834. DOI https://doi.org/10.1016/j.chemosphere.2005.07.066
Dernie, K.M., Kaiser, M.J., Richardson, E.A. & Warwick, R.M., 2003. Recovery of soft sediment communities and habitats following physical disturbance. Journal of Experimental Marine Biology and Ecology, 285-286, 415-434.
Dewarumez, J-M., Smigielski, F. & Richard, A., 1976. Abra alba (mollusque lamellibranche) sa localisation en zone littorale de la mer du Nord. Haliotis, 7, 13-19.
Diaz, R.J. & Rosenberg, R., 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology: an Annual Review, 33, 245-303.
Duineveld, G.C.A. & Jenness, M.I., 1984. Differences in growth rates of the sea urchin Echinocardium cordatum as estimated by the parameters of the von Bertalanffy equation applied to skeletal rings. Marine Ecology Progress Series, 19, 64-72.
Eagle, R.A., 1975. Natural fluctuations in a soft bottom benthic community. Journal of the Marine Biological Association of the United Kingdom, 55, 865-878.
Eleftheriou, A. & Robertson, M.R., 1992. The effects of experimental scallop dredging on the fauna and physical environment of a shallow sandy community. Netherlands Journal of Sea Research, 30, 289-299.
Emerson, C.W. & Grant, J., 1991. The control of soft-shell clam (Mya arenaria) recruitment on intertidal sandflats by bedload sediment transport. Limnology and Oceanography, 36, 1288-1300.
Essink, K., 1999. Ecological effects of dumping of dredged sediments; options for management. Journal of Coastal Conservation, 5, 69-80.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
FitzGerald, A., 2007. Slipper Limpet Utilisation and Management. Final Report. Port of Truro Oyster Management Group., Truro, 101 pp. Available from https://www.shellfish.org.uk/files/Literature/Projects-Reports/0701-Slipper_Limpet_Report_Final_Small.pdf
Fletcher, S., Saunders, J. & Herbert, R.J., 2011. A review of the ecosystem services provided by broad-scale marine habitats in England's MPA network. Journal of Coastal Research, 64, 378.
Forrest, B. M., Keeley, N.B., Hopkins, G.A., Webb, S.C. & Clement, D.M., 2009. Bivalve aquaculture in estuaries: Review and synthesis of oyster cultivation effects. Aquaculture 298 (1–2), 1-15.
Gerdes, D., 1977. The re-establishment of an Amphiura filiformis (O.F. Müller) population in the inner part of the German Bight. In Biology of Benthic Organisms (ed. B. Keegan et al.), pp. 277-284. Oxford: Pergamon Press.
Gittenberger, A. & Van Loon, W.M.G.M., 2011. Common marine macrozoobenthos species in the Netherlands, their characteristics and sensitivities to environmental pressures. GiMaRIS Report no 2011.08. DOI: https://doi.org/10.13140/RG.2.1.3135.7521
Glémarec, M., 1973. The benthic communities of the European North Atlantic continental shelf. Oceanography and Marine Biology: an Annual Review, 11, 263-289.
Glémarec, M., 1979. Problemes d'ecologie dynamique et de succession en baie de Concarneau. Vie et Milieu, 28-29, 1-20.
Gogina, M., Glockzin, M. & Zettler, M.L., 2010a. Distribution of benthic macrofaunal communities in the western Baltic Sea with regard to near-bottom environmental parameters. 1. Causal analysis. Journal of Marine Systems, 79 (1), 112-123.
Guillou, J., 1985. Population dynamics of Echinocardium cordatum (Pennant) in the bay of Douarnenez (Brittany). In Proceedings of the Fifth International Echinocardium Conference / Galway / 24-29 September 1984 (ed. B.F. Keegan & B.D.S. O'Conner), 275-280. Rotterdam: Balkema.
Gunnarsson, J.S. & Skold, M., 1999. Accumulation of polychlorinated biphenyls by the infaunal brittle stars Amphiura filiformis and A. chiajei: effects of eutrophication and selective feeding. Marine Ecology Progress Series, 186, 173-185.
Hagen, N., 1997. Sea urchin outbreaks and epizootic disease as regulating mechanisms in coastal ecosystems. Oceanographic Literature Review, 2 (44), 131.
Hall, K., Paramour, O.A.L., Robinson, L.A., Winrow-Giffin, A., Frid, C.L.J., Eno, N.C., Dernie, K.M., Sharp, R.A.M., Wyn, G.C. & Ramsay, K., 2008. Mapping the sensitivity of benthic habitats to fishing in Welsh waters - development of a protocol. CCW (Policy Research) Report No: 8/12, Countryside Council for Wales (CCW), Bangor, 85 pp.
Hall, S.J., 1994. Physical disturbance and marine benthic communities: life in unconsolidated sediments. Oceanography and Marine Biology: an Annual Review, 32, 179-239.
Hargrave, B.T., 1980. Factors affecting the flux of organic matter to sediments in a marine bay. In Marine Benthic Dynamics (eds. Tenore, K.R. & Coull, B.C.), 243-263. USA: University of South Carolina Press.
Hartnoll, R.G., 1998. Circalittoral faunal turf biotopes: an overview of dynamics and sensitivity characteristics for conservation management of marine SACs, Volume VIII. Scottish Association of Marine Sciences, Oban, Scotland, 109 pp. [UK Marine SAC Project. Natura 2000 reports.] Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/circfaun.pdf
Harvey, R. & Gage, J.D., 1995. Reproduction and recruitment of Nuculoma tenuis (Bivalvia: Nuculoida) from Loch Etive, Scotland. Journal of Molluscan Studies, 61(4), 409-419.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hernroth, B., Sköld, H.N., Wiklander, K., Jutfelt, F. & Baden, S., 2012. Simulated climate change causes immune suppression and protein damage in the crustacean Nephrops norvegicus. Fish & Shellfish Immunology, 33 (5), 1095-1101.
Hiddink, J.G., Jennings, S., Kaiser, M.J., Queirós, A.M., Duplisea, D.E. & Piet, G.J., 2006. Cumulative impacts of seabed trawl disturbance on benthic biomass, production, and species richness in different habitats. Canadian Journal of Fisheries and Aquatic Sciences, 63 (4), 721-736.
Hill, J.M. & Wilson, E. 2008. Amphiura filiformis A brittlestar. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. Available from: http://192.171.193.68/species/detail/1400
Hily, C. & Le Bris, H., 1984. Dynamics of an Abra alba population (Bivalve: Scrobiculariidae) in the Bay of Brest. Estuarine and Coastal Shelf Science, 19, 463-475.
Hinchey, E.K., Schaffner, L.C., Hoar, C.C., Vogt, B.W. & Batte, L.P., 2006. Responses of Estuarine Benthic Invertebrates to Sediment Burial: The Importance of Mobility and Adaptation. Hydrobiologia, 556 (1), 85-98.
Hinz, H., Capasso, E., Lilley, M., Frost, M. & Jenkins, S.R., 2011. Temporal differences across a bio-geographical boundary reveal slow response of sub-littoral benthos to climate change. Marine Ecology Progress Series, 423, 69-82. DOI https://doi.org/10.3354/meps08963
Hinz, H., Prieto, V. & Kaiser, M.J., 2009. Trawl disturbance on benthic communities: chronic effects and experimental predictions. Ecological Applications 19 (3), 761-773.
Hiscock, K., 1983. Water movement. In Sublittoral ecology. The ecology of shallow sublittoral benthos (ed. R. Earll & D.G. Erwin), pp. 58-96. Oxford: Clarendon Press.
Hiscock, K., 1984. Rocky shore surveys of the Isles of Scilly. March 27th to April 1st and July 7th to 15th 1983. Peterborough: Nature Conservancy Council, CSD Report, No. 509.
Hiscock, K., ed. 1998. Marine Nature Conservation Review. Benthic marine ecosystems of Great Britain and the north-east Atlantic. Peterborough, Joint Nature Conservation Committee.
Hiscock, K., Langmead, O., Warwick, R. & Smith, A., 2005. Identification of seabed indicator species to support implementation of the EU Habitats and Water Framework Directives. Report to the Joint Nature Conservation Committee and the Environment Agency The Marine Biological Association, Plymouth, 77 pp.
Hoare, R. & Wilson, E.H., 1977. Observations on the behaviour and distribution of Virgularia mirabilis O.F. Müller (Coelenterata: Pennatulacea) in Holyhead harbour. In Proceedings of the Eleventh European Symposium on Marine Biology, University College, Galway, 5-11 October 1976. Biology of Benthic Organisms, (ed. B.F. Keegan, P.O. Ceidigh & P.J.S. Boaden, pp. 329-337. Oxford: Pergamon Press. Oxford: Pergamon Press.
Holme, N., 1967. Changes in the bottom fauna of Weymouth Bay and Poole Bay following the severe winter of 1962–63. Journal of the Marine Biological Association of the United Kingdom 47 (02), 397-405
Holte, B., Oug, E. & Dahle, S., 2005. Soft-bottom fauna and oxygen minima in sub-arctic north Norwegian marine sill basins. Marine Biology Research, 1 (2), 85-96.
Holtmann, S.E., Groenewold, A., Schrader, K.H.M., Asjes, J., Craeymeersch, J.A., Duineveld, G.C.A., van Bostelen, A.J. & van der Meer, J., 1996. Atlas of the zoobenthos of the Dutch continental shelf. Rijswijk: Ministry of Transport, Public Works and Water Management.
Howson, C.M., Connor, D.W. & Holt, R.H.F., 1994. The Scottish sealochs - an account of surveys undertaken for the Marine Nature Conservation Review. Joint Nature Conservation Committee Report, No. 164 (Marine Nature Conservation Review Report MNCR/SR/27)., Joint Nature Conservation Committee Report, No. 164 (Marine Nature Conservation Review Report MNCR/SR/27).
Hughes, D.J. & Atkinson, R.J.A., 1997. A towed video survey of megafaunal bioturbation in the north-eastern Irish Sea. Journal of the Marine Biological Association of the United Kingdom, 77, 635-653.DOI https://doi.org/10.1017/s0025315400036122
Hutchins, D.A., Teyssié, J-L., Boisson, F., Fowler, S.W., & Fisher, N.S., 1996. Temperature effects on uptake and retention of contaminant radionuclides and trace metals by the brittle star Ophiothrix fragilis. Marine Environmental Research, 41, 363-378.
Huthnance, J., 2010. Ocean Processes Feeder Report. London, DEFRA on behalf of the United Kingdom Marine Monitoring and Assessment Strategy (UKMMAS) Community.
Hylland, K., Sköld, M., Gunnarsson, J.S. & Skei, J., 1996. Interactions between eutrophication and contaminants. IV. Effects on sediment-dwelling organisms. Marine Pollution Bulletin, 33, 90-99.
Jackson, A. 2007. Thyasira gouldi Northern hatchet shell. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. Available from: http://www.marlin.ac.uk/species/detail/1149
Jennings, S. & Kaiser, M.J., 1998. The effects of fishing on marine ecosystems. Advances in Marine Biology, 34, 201-352.
Jennings, S., Dinmore, T.A., Duplisea, D.E., Warr, K.J. & Lancaster, J.E., 2001. Trawling disturbance can modify benthic production processes. Journal of Animal Ecology, 70 (3), 459-475.
JNCC (Joint Nature Conservation Committee), 2022. The Marine Habitat Classification for Britain and Ireland Version 22.04. [Date accessed]. Available from: https://mhc.jncc.gov.uk/
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Jones, L.A., Hiscock, K. & Connor, D.W., 2000. Marine habitat reviews. A summary of ecological requirements and sensitivity characteristics for the conservation and management of marine SACs. Joint Nature Conservation Committee, Peterborough. (UK Marine SACs Project report.). Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/marine-habitats-review.pdf
Jones, N.S., 1951. The bottom fauna of the south of the Isle of Man. Journal of Animal Ecology, 20, 132-144.
Josefson, A., 1982. Regulation of population size, growth, and production of a deposit-feeding bivalve: a long-term field study of three deep-water populations off the Swedish west coast. Journal of Experimental Marine Biology and Ecology, 59 (2), 125-150.
Josefson, A.B. & Smith, S., 1984. Changes of benthos-biomass in the Skagerrak-Kattegat during the 70s: a result of chance events, climatic changes or eutrophication? Meddelande fran Havsfiskelaboratoriet. Lysekil, 292, 111-121.
Josefson, A.B., 1990. Increase in the benthic biomass in the Skagerrak-Kattegat during the 1970s and 1980s - effects of organic enrichment? Marine Ecology Progress Series, 66, 117-130.
Künitzer, A., Basford, D., Craeymeersch, J.A., Dewarumez, J.M., Derjes, J., Duinevald, G.C.A., Eleftheriou, A., Heip, C, Herman, P., Kingston, P., Neirmann, U., Rachor, E., Rumohr, H. & Wilde, P.A.J. de, 1992. The benthic infauna of the North Sea: species distribution and assemblages. ICES Journal of Marine Science, 49, 127-143.
Kaiser, M., Clarke, K., Hinz, H., Austen, M., Somerfield, P. & Karakassis, I., 2006. Global analysis of response and recovery of benthic biota to fishing. Marine Ecology Progress Series, 311, 1-14.
Kashenko, S.D., 1994. Larval development of the heart urchin Echinocardium cordatum feeding on different macroalgae. Biologiya Morya, 20, 385-389.
Klein, R. & Witbaard, R., 1993. The appearance of scars on the shell of Arctica islandica L. (Mollusca, Bivalvia) and their relation to bottom trawl fishery. NIOZ - Rapport, 12., Unpublished, Nederlands Instituut voor Onderzoek der Zee.
Kröncke, I., Reiss, H. & Dippner, J.W., 2013a. Effects of cold winters and regime shifts on macrofauna communities in shallow coastal regions. Estuarine, Coastal and Shelf Science, 119, 79-90.
Kröncke, I., Reiss, H., Eggleton, J.D., Aldridge, J., Bergman, M.J. N., Cochrane, S., Craeymeersch, J.A., Degraer, S., Desroy, N., Dewarumez, J., Duineveld, G.C. A., Essink, K., Hillewaert, H., Lavaleye, M.S.S., Moll, A., Nehring, S., Newell, R., Oug, E., Pohlmann, T., Rachor, E., Robertson, M., Rumohr, H., Schratzberger, M., Smith, R., Berghe, E.V., Van Dalfsen, J., Van Hoey, G., Vincx, M., Willems, W. & Rees, H.L., 2011. Changes in North Sea macrofauna communities and species distribution between 1986 and 2000. Estuarine, Coastal and Shelf Science, 94 (1), 1-15.
Künitzer, A., 1989. Factors affecting the population dynamics of Amphiura filiformis (Echinodermata: Ophiuroidea) and Mysella bidentata (Bivalvia: Galeommatacea) in the North Sea. In Reproduction, genetics and distributions of marine organisms. 23rd European Marine Biology Symposium (ed. J.S. Ryland and P.A. Tyler), pp. 395-406. Denmark: Olsen and Olsen.
Kutti, T., Ervik, A. & Høisæter, T., 2008. Effects of organic effluents from a salmon farm on a fjord system. III. Linking deposition rates of organic matter and benthic productivity. Aquaculture, 282 (1), 47-53.
Larsen, J.B., Frischer, M.E., Ockelmann, K.W., Rasmussen, L.J. & Hansen, B.W., 2007. Temporal occurrence of planktotrophic bivalve larvae identified morphologically and by single step nested multiplex PCR. Journal of Plankton Research, 29 (5), 423-436.
Last, K.S., Hendrick V. J, Beveridge C. M & Davies A. J, 2011. Measuring the effects of suspended particulate matter and smothering on the behaviour, growth and survival of key species found in areas associated with aggregate dredging. Report for the Marine Aggregate Levy Sustainability Fund, Project MEPF 08/P76, 69 pp.
Lawrence, J. M., 2010. Energetic costs of loss and regeneration of arms in stellate echinoderms. Integrative and Comparative Biology 50 (4), 506-514.
Lawrence, J.M., 1996. Mass mortality of echinoderms from abiotic factors. In Echinoderm Studies Vol. 5 (ed. M. Jangoux & J.M. Lawrence), pp. 103-137. Rotterdam: A.A. Balkema.
Lebour, M.V., 1938. Notes on the breeding of some lamellibranchs from Plymouth and their larvae. Journal of the Marine Biological Association of the United Kingdom, 23, 119-144.
Lessios, H., 1988. Mass mortality of Diadema antillarum in the Caribbean: what have we learned? Annual Review of Ecology and Systematics, 19, 371-393.
Lindley, J.A., Gamble, J.C. & Hunt, H.G., 1995. A change in the zooplankton of the central North Sea (55° to 58° N): a possible consequence of changes in the benthos. Marine Ecology Progress Series, 119, 299-303.
Loizeau, V. & Menesguen, A., 1993. A steady-state model of PCB accumulation in a dab, Limanda limanda, food web. Oceanologica Acta, 16, 633-640.
Long, D., 2006. BGS detailed explanation of seabed sediment modified Folk classification. Available from: http://www.emodnet-seabedhabitats.eu/PDF/GMHM3_Detailed_explanation_of_seabed_sediment_classification.pdf
López-Jamar, E., González, J. & Mejuto, J., 1987. Ecology, growth and production of Thyasira flexuosa (Bivalvia, Lucinacea) from Ría de la Coruña, North-west Spain. Ophelia, 27, 111-126.
Møhlenberg, F. & Kiørboe, T., 1983. Burrowing and avoidance behaviour in marine organisms exposed to pesticide-contaminated sediment. Marine Pollution Bulletin, 14 (2), 57-60.
Mackie, A.S.Y., 1990. Offshore benthic communities of the Irish Sea. In The Irish Sea: an environmental review. Part 1: nature conservation, ed. Irish Sea Study Group, pp. 169-218. Liverpool, Liverpool University Press for Irish Sea Study Group.
Macleod, C.K., Moltschaniwskyj, N.A. & Crawford, C.M., 2008. Ecological and functional changes associated with long-term recovery from organic enrichment. Marine Ecology Progress Series, 365, 17-24.
Marshall, C.E. 2005. Mysella bidentata and Thyasira spp. in circalittoral muddy mixed sediment. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. Available from: http://www.marlin.ac.uk/habitat/detail/374
Maurer, D., Keck, R.T., Tinsman, J.C. & Leathem, W.A., 1982. Vertical migration and mortality of benthos in dredged material: Part III—Polychaeta. Marine Environmental Research, 6 (1), 49-68. DOI https://doi.org/10.1016/0141-1136(82)90007-1
Maurer, D., Keck, R.T., Tinsman, J.C., Leatham, W.A., Wethe, C., Lord, C. & Church, T.M., 1986. Vertical migration and mortality of marine benthos in dredged material: a synthesis. Internationale Revue der Gesamten Hydrobiologie, 71, 49-63. DOI https://doi.org/10.1002/iroh.19860710106
McNeill, G., Nunn, J. & Minchin, D., 2010. The slipper limpet Crepidula fornicata Linnaeus, 1758 becomes established in Ireland. Aquatic Invasions, 5 (Suppl. 1), S21-S25. DOI https://doi.org/10.3391/ai.2010.5.S1.006
Menesguen, A. & Gregoris, T., 2018. Modelling benthic invasion by the colonial gastropod Crepidula fornicata and its competition with the bivalve Pecten maximus. 1. A new 0D model for population dynamics of colony-forming species. Ecological Modelling, 368, 277-287. DOI https://doi.org/10.1016/j.ecolmodel.2017.12.005
MES, 2010. Marine Macrofauna Genus Trait Handbook. Marine Ecological Surveys Limited. http://www.genustraithandbook.org.uk/
Morton, J.W., 1976. Ecological impacts of dredging and dredge spoil disposal: A literature review. M. S. thesis, Cornell University, Ithaca, N. Y..
Muus, K., 1981. Density and growth of juvenile Amphiura filiformis (Ophiuroidea) in the Oresund. Ophelia, 20, 153-168.
Newell, R.C., Seiderer, L.J. & Hitchcock, D.R., 1998. The impact of dredging works in coastal waters: a review of the sensitivity to disturbance and subsequent biological recovery of biological resources on the sea bed. Oceanography and Marine Biology: an Annual Review, 36, 127-178.
Newton, L.C. & McKenzie, J.D., 1995. Echinoderms and oil pollution: a potential stress assay using bacterial symbionts. Marine Pollution Bulletin, 31, 453-456.
Nickell, L.A. & Atkinson, R.J.A., 1995. Functional morphology of burrows and trophic modes of three thalassinidean shrimp species, and a new approach to the classification of thalassinidean burrow morphology. Marine Ecology Progress Series, 128, 181-197.
Niermann, U., 1997. Macrobenthos of the south-eastern North Sea during 1983-1988. Berichte der Biologischen Anstalt Helgoland, 13, 144pp.
Nilsson, H.C. & Rosenberg, R., 1994. Hypoxic response of two marine benthic communities. Marine Ecology Progress Series, 115, 209-217. DOI https://doi.org/10.3354/meps115209
Nilsson, H.C. & Skold, M., 1996. Arm regeneration and spawning in the brittle star Amphiura filiformis (O.F. Müller) during hypoxia. Journal of Experimental Marine Biology and Ecology, 199, 193-206.
Nilsson, H.C., 1999. Effects of hypoxia and organic enrichment on growth of the brittle star Amphiura filiformis (O.F. Müller) and Amphiura chaijei Forbes. Journal of Experimental Marine Biology and Ecology, 237, 11-30.
O'Brien, K. & Keegan, B., 2006. Age-related reproductive biology of the bivalve Mysella bidentata (Montagu)(Bivalvia: Galeommatacea) in Kinsale Harbour (South coast of Ireland). The Irish Naturalists' Journal, 28 (7), 284-299.
O'Connor, B., Bowmer, T. & Grehan, A., 1983. Long-term assessment of the population dynamics of Amphiura filiformis (Echinodermata: Ophiuroidea) in Galway Bay (west coast of Ireland). Marine Biology, 75, 279-286.
O'Connor, B., Bowmer, T., McGrath, D. & Raine, R., 1986. Energy flow through an Amphiura filiformis (Ophiuroidea: Echinodermata) in Galway Bay, west coast of Ireland: a preliminary investigation. Ophelia, 26, 351-357.
O'Foighill, D., McGrath, D., Conneely, M.E., Keegan, B.F. & Costelloe, M., 1984. Population dynamics and reproduction of Mysella bidentata (Bivalvia: Galeommatacea) in Galway Bay, Irish west coast. Marine Biology, 81, 283-291.
OBIS 2014. Data from the Ocean Biogeographic Information System. Intergovernmental Oceanographic Commission of UNESCO. [online]. Available from: http://www.iobis.org
Ockelmann, K.W. & Muus, K., 1978. The biology, ecology and behaviour of the bivalve Mysella bidentata (Montagu). Ophelia, 17, 1-93.
Olivier, F., Vallet, C., Dauvind, J-C. & Retière, C., 1996. Drifting in post-larvae and juveniles in an Abra alba (Wood) community of the eastern part of the Bay of Seine (English Channel). Journal of Experimental Marine Biology and Ecology, 199, 89-109.
Olsgard, F. & Gray, J.S., 1995. A comprehensive analysis of the effects of offshore oil and gas exploration and production on the benthic communities of the Norwegian continental shelf. Marine Ecology Progress Series, 122, 277-306.
OSPAR Commission. 2009. Background document for Modiolus modiolus beds. OSPAR Commission Biodiversity Series. OSPAR Commission: London. Available from: http://www.ospar.org/documents?v=7193
Pagett, R.M., 1981. The penetration of brackish-water by the Echinodermata. In Feeding and Survival Strategies of Estuarine Organisms (ed. N.V. Jones & W.J. Wolff), 15, 135-151. New York: Plenum Press.
Pearson, T.H. & Rosenberg, R., 1976. A comparative study of the effects on the marine environment of wastes from cellulose industries in Scotland and Sweden. Ambio, 5, 77-79.
Pearson, T.H. & Rosenberg, R., 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology: an Annual Review, 16, 229-311.
Pedrotti, M.L., 1993. Spatial and temporal distribution and recruitment of echinoderm larvae in the Ligurian Sea. Journal of the Marine Biological Association of the United Kingdom, 73, 513-530.
Peterson, C.H., 1977. Competitive organisation of the soft bottom macrobenthic communities of southern California lagoons. Marine Biology, 43, 343-359.
Picton, B.E. & Costello, M.J., 1998. BioMar biotope viewer: a guide to marine habitats, fauna and flora of Britain and Ireland. [CD-ROM] Environmental Sciences Unit, Trinity College, Dublin.
Picton, B.E., Emblow, C.S., Morrow, C.C., Sides, E.M. & Costello, M.J., 1994b. Marine communities of the Mulroy Bay and Lough Swill area, north-west Ireland, with an assessment of their nature conservation importance. , Unpublished, Environmental Sciences Unit, Trinity College. (Field Survey Report).
Powell-Jennings, C. & Callaway, R., 2018. The invasive, non-native slipper limpet Crepidula fornicata is poorly adapted to sediment burial. Marine Pollution Bulletin, 130, 95-104. DOI https://doi.org/10.1016/j.marpolbul.2018.03.006
Powilleit, M., Graf, G., Kleine, J., Riethmuller, R., Stockmann, K., Wetzel, M.A. & Koop, J.H.E., 2009. Experiments on the survival of six brackish macro-invertebrates from the Baltic Sea after dredged spoil coverage and its implications for the field. Journal of Marine Systems, 75 (3-4), 441-451.
Preston, J., Fabra, M., Helmer, L., Johnson, E., Harris-Scott, E. & Hendy, I.W., 2020. Interactions of larval dynamics and substrate preference have ecological significance for benthic biodiversity and Ostrea edulis Linnaeus, 1758 in the presence of Crepidula fornicata. Aquatic Conservation: Marine and Freshwater Ecosystems, 30 (11), 2133-2149. DOI https://doi.org/10.1002/aqc.3446
Probert, P.K., 1981. Changes in the benthic community of china clay waste deposits is Mevagissey Bay following a reduction of discharges. Journal of the Marine Biological Association of the United Kingdom, 61, 789-804. Doi https://doi.org/10.1017/S0025315400048219
Ragueneau, O., Raimonet, M., Maze, C., Coston-Guarini, J., Chauvaud, L., Danto, A., Grall, J., Jean, F., Paulet, Y. M. & Thouzeau, G., 2018. The Impossible Sustainability of the Bay of Brest? Fifty Years of Ecosystem Changes, Interdisciplinary Knowledge Construction and Key Questions at the Science-Policy-Community Interface. Frontiers in Marine Science, 5. DOI https://doi.org/10.3389/fmars.2018.00124
Ramsay, K., Kaiser, M.J. & Hughes, R.N. 1998. The responses of benthic scavengers to fishing disturbance by towed gears in different habitats. Journal of Experimental Marine Biology and Ecology, 224, 73-89.
Rees, E.I.S., Nicholaidou, A. & Laskaridou, P., 1977. The effects of storms on the dynamics of shallow water benthic associations. In Proceedings of the 11th European Symposium on Marine Biology, Galway, Ireland, October 5-11, 1976. Biology of Benthic Organisms, (ed. B.F. Keegan, P. O'Ceidigh & P.J.S. Boaden), pp. 465-474.
Rees, H.L. & Dare, P.J., 1993. Sources of mortality and associated life-cycle traits of selected benthic species: a review. MAFF Fisheries Research Data Report, no. 33., Lowestoft: MAFF Directorate of Fisheries Research.
Reiss, H., Greenstreet, S.P., Sieben, K., Ehrich, S., Piet, G.J., Quirijns, F., Robinson, L., Wolff, W.J. & Kröncke, I., 2009. Effects of fishing disturbance on benthic communities and secondary production within an intensively fished area. Marine Ecology Progress Series, 394, 201-213.
Ridder de, C., David, B., Laurin, B. & Gall le, P., 1991. Population dynamics of the spatangoid echinoid Echinocardium cordatum (Pennant) in the Bay of Seine, Normandy. In Proceedings of the Seventh International Echinoderm Conference Atami, 9 - 14 September 1991: Biology of Echinodermata, (ed. Yanagisawa, T., Yasumasu, I., Oguro, C., Suzuki, N. & Motokawa, T.), 153-158. Balkema, Rotterdam.
Roberts, C., Smith, C., H., T. & Tyler-Walters, H., 2010. Review of existing approaches to evaluate marine habitat vulnerability to commercial fishing activities. Report to the Environment Agency from the Marine Life Information Network and ABP Marine Environmental Research Ltd. Environment Agency Evidence Report: SC080016/R3., Environment Agency, Peterborough, pp. http://publications.environment-agency.gov.uk/PDF/SCHO1110BTEQ-E-E.pdf
Rosenberg, R., 1995. Benthic marine fauna structured by hydrodynamic processes and food availability. Netherlands Journal of Sea Research, 34, 303-317.
Rosenberg, R., Gray, J.S., Josefson, A.B. & Pearson, T.H., 1987. Petersen's benthic stations revisited. II. Is the Oslofjord and eastern Skagerrak enriched? Journal of Experimental Marine Biology and Ecology, 105, 219-251. DOI https://doi.org/10.1016/0022-0981(87)90174-2
Rosenberg, R., Hellman, B. & Johansson, B., 1991. Hypoxic tolerance of marine benthic fauna. Marine Ecology Progress Series, 79, 127-131. DOI https://dx.doi.org/10.3354/meps079127
Rosenberg, R., Nilsson, H.C., Hollertz, K. & Hellman, B., 1997. Density-dependent migration in an Amphiura filiformis (Amphiuridae, Echinodermata) infaunal population. Marine Ecology Progress Series, 159, 121-131.
Rouse, G.W. & Pleijel, F., 2001. Polychaetes. New York: Oxford University Press.
Rowden, A.A. & Jones, M.B., 1997. Recent mud shrimp burrows and bioturbation. Porcupine Newsletter, 6, 153-158.
Rowden, A.A., Jones, M.B. & Morris, A.W., 1998. The role of Callianassa subterranea (Montagu) (Thalassinidea) in sediment resuspension in the North Sea. Continental Shelf Research, 18, 1365-1380.
Rumohr, H. & Kujawski, T., 2000. The impact of trawl fishery on the epifauna of the southern North Sea. ICES Journal of Marine Science, 57, 1389-1394.
Russell, M., 2013. Echinoderm Responses to Variation in Salinity. Advances in Marine Biology, 66, 171-212. DOI http://dx.doi.org/10.1016/B978-0-12-408096-6.00003-1
Rygg, B., 1985. Effect of sediment copper on benthic fauna. Marine Ecology Progress Series, 25, 83-89.
Sanders, H.L., 1978. Florida oil spill impact on the Buzzards Bay benthic fauna: West Falmouth. Journal of the Fisheries Board of Canada, 35 (5), 717-730.
Sbaihat, M., Reyati, S. & Al-Najjar, T., 2013. Levels of heavy metals in Ophoroidea (Ophiocoma scolopendrina) from the Gulf of Aqaba, Red Sea. Fresenius Environmental Bulletin, 22 (12), 3519-3524.
Schückel, U., Ehrich, S. & Kröncke, I., 2010. Temporal variability of three different macrofauna communities in the northern North Sea. Estuarine, Coastal and Shelf Science, 89 (1), 1-11.
Shaffer, P.L., 1983. Population ecology of Heteromastus filiformis (polychaeta: capitallidae). Netherlands Journal of Sea Research, 17(1), 106-125.
Sherk Jr, J.A. & Cronin, L.E., 1970. The effects of suspended and deposited sediments on estuarine organisms. Literature summary and research needs, Contr. 443, Natural Resources Institute, University of Maryland.
Sigurdsson, J.B., Titman, C.W. & Davies, P.A., 1976. The dispersal of young post-larval bivalve molluscs by byssus threads. Nature, 262, 386-387.
Sinderman, C.J., 1990. Principle diseases of marine fish and shellfish, 2nd edition, Volume 2. Diseases of marine shellfish. Academic Press, 521 pp.
Sköld, M. & Gunnarsson, J.S.G., 1996. Somatic and germinal growth of the infaunal brittle stars Amphiura filiformis and A. chiajei in response to organic enrichment. Marine Ecology Progress Series, 142, 203-214.
Sköld, M., 1998. Escape responses in four epibenthic brittle stars (Ophiuroidea: Echinodermata). Ophelia, 49, 163-179.
Sköld, M., Loo, L. & Rosenberg, R., 1994. Production, dynamics and demography of an Amphiura filiformis population. Marine Ecology Progress Series, 103, 81-90.
Smith, J.E. (ed.), 1968. 'Torrey Canyon'. Pollution and marine life. Cambridge: Cambridge University Press.
Sparks-McConkey, P.J. & Watling, L., 2001. Effects on the ecological integrity of a soft-bottom habitat from a trawling disturbance. Hydrobiologia, 456, 73-85.
Stachowitsch, M., 1984. Mass mortality in the Gulf of Trieste: the course of community destruction. Marine Ecology, Pubblicazione della Statione Zoologica di Napoli, 5, 243-264.
Stickle, W.B. & Diehl, W.J., 1987. Effects of salinity on echinoderms. In Echinoderm Studies, Vol. 2 (ed. M. Jangoux & J.M. Lawrence), pp. 235-285. A.A. Balkema: Rotterdam.
Stiger-Pouvreau, V. & Thouzeau, G., 2015. Marine Species Introduced on the French Channel-Atlantic Coasts: A Review of Main Biological Invasions and Impacts. Open Journal of Ecology, 5, 227-257. DOI https://doi.org/10.4236/oje.2015.55019
Suchanek, T.H., 1993. Oil impacts on marine invertebrate populations and communities. American Zoologist, 33, 510-523. DOI https://doi.org/10.1093/icb/33.6.510
Sundborg, Å., 1956. The River Klarälven: a study of fluvial processes. Geografiska Annaler, 38 (2), 125-237.
Tebble, N., 1976. British Bivalve Seashells. A Handbook for Identification, 2nd ed. Edinburgh: British Museum (Natural History), Her Majesty's Stationary Office.
Tenore, K.R., 1988. Nitrogen in benthic food chains. In Nitrogen Cycling in Coastal Marine Environments, (eds. Blackburn, T.H. & Sörensen J.), 191-206. New York: John Wiley & Sons Ltd.
Thieltges, D.W., Strasser, M. & Reise, K., 2003. The American slipper-limpet Crepidula fornicata (L.) in the Northern Wadden Sea 70 years after its introduction. Helgoland Marine Research, 57, 27-33
Thieltges, D.W., Strasser, M., Van Beusekom, J.E. & Reise, K., 2004. Too cold to prosper—winter mortality prevents population increase of the introduced American slipper limpet Crepidula fornicata in northern Europe. Journal of Experimental Marine Biology and Ecology, 311 (2), 375-391. DOI https://doi.org/10.1016/j.jembe.2004.05.018
Thorson, G., 1946. Reproduction and larval development of Danish marine bottom invertebrates, with special reference to the planktonic larvae in the Sound (Øresund). Meddelelser fra Kommissionen for Danmarks Fiskeri- Og Havundersögelser, Serie: Plankton, 4, 1-523.
Thorson, G., 1950. Reproductive and larval ecology of marine bottom invertebrates. Biological Reviews, 25, 1-45.
Thouzeau, Gérard, Chauvaud, Laurent, Grall, Jacques & Guérin, Laurent, 2000. Rôle des interactions biotiques sur le devenir du pré-recrutement et la croissance de Pecten maximus (L.) en rade de Brest. Comptes Rendus de l#&39;Académie des Sciences - Series III - Sciences de la Vie, 323 (9), 815-825. DOI https://doi.org/10.1016/S0764-4469(00)01232-4
Thouzeau, G., Chavaud, L., Grall, J. & Guerin, L., 2000. Do biotic interactions control pre-recruitment and growth of Pecten maximus (L.) in the Bay of Brest ? Comptes rendus - acadamies des sciences, Paris, 323, 815-825.
Tillin, H. & Tyler-Walters, H., 2014b. Assessing the sensitivity of subtidal sedimentary habitats to pressures associated with marine activities. Phase 2 Report – Literature review and sensitivity assessments for ecological groups for circalittoral and offshore Level 5 biotopes. JNCC Report No. 512B, 260 pp. Available from: www.marlin.ac.uk/publications
Tillin, H.M., Kessel, C., Sewell, J., Wood, C.A. & Bishop, J.D.D., 2020. Assessing the impact of key Marine Invasive Non-Native Species on Welsh MPA habitat features, fisheries and aquaculture. NRW Evidence Report. Report No: 454. Natural Resources Wales, Bangor, 260 pp. Available from https://naturalresourceswales.gov.uk/media/696519/assessing-the-impact-of-key-marine-invasive-non-native-species-on-welsh-mpa-habitat-features-fisheries-and-aquaculture.pdf
Trannum, H. C., Nilsson, H.C., Schaanning, M.T. & Øxnevad, S., 2010. Effects of sedimentation from water-based drill cuttings and natural sediment on benthic macrofaunal community structure and ecosystem processes. Journal of Experimental Marine Biology and Ecology 383 (2), 111-121.
Vaquer-Sunyer, R. & Duarte, C.M., 2008. Thresholds of hypoxia for marine biodiversity. Proceedings of the National Academy of Sciences, 105 (40), 15452-15457.DOI https://doi.org/10.1073/pnas.0803833105
Viñas, L., Franco, M.A., Soriano, J.A., González, J.J., Ortiz, L., Bayona, J.M. & Albaigés, J., 2009. Accumulation trends of petroleum hydrocarbons in commercial shellfish from the Galician coast (NW Spain) affected by the Prestige oil spill. Chemosphere, 75 (4), 534-541.
Vistisen, B. & Vismann, B., 1997. Tolerance to low oxygen and sulfide in Amphiura filiformis and Ophiura albida (Echinodermata: Ophiuroidea). Marine Biology, 128, 241-246.
Walsh, G.E., McLaughlin, L.L., Louie, M.K., Deans, C.H. & Lores, E.M., 1986. Inhibition of arm regeneration by Ophioderma brevispina (Echinodermata: Ophiuroidea) by tributyltin oxide and triphenyltin oxide. Ecotoxicology and Environmental Safety, 12, 95-100.
Widdows, J., Bayne, B.L., Livingstone, D.R., Newell, R.I.E. & Donkin, P., 1979. Physiological and biochemical responses of bivalve molluscs to exposure to air. Comparative Biochemistry and Physiology, 62A, 301-308.
Wilson, J.G., 1981. Temperature tolerance of circatidal bivalves in relation to their distribution. Journal of Thermal Biology, 6, 279-286.
Wilson, J.G., 1992. Age specific energetics of reproduction in Nucula turgida (Leckenby & Marshall) a bivalve with lecithotrophic larval development. Invertebrate Reproduction and Development, 22, 275-280.
Wolff, W.J., 1968. The Echinodermata of the estuarine region of the rivers Rhine, Meuse and Scheldt, with a list of species occurring in the coastal waters of the Netherlands. The Netherlands Journal of Sea Research, 4, 59-85.
Citation
This review can be cited as:
Last Updated: 07/09/2023