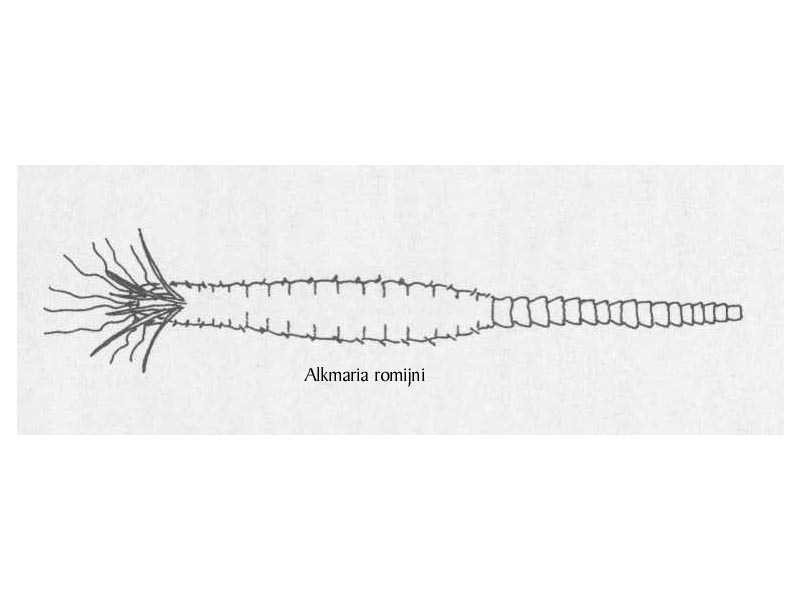
Tentacled lagoon worm (Alkmaria romijni)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Dr Harvey Tyler-Walters & Nicola White | Refereed by | Dr Paul M. Gilliland |
| Authority | Horst, 1919 | ||
| Other common names | - | Synonyms | - |
Summary
Description
A small worm, up to 5 mm long, with eight tentacles that are thread-like and slimy. It has six gills that are banded by rings of greenish-grey pigment.
Recorded distribution in Britain and Ireland
Recorded on the southern shores of the North Sea as far north as the Humber, along the English Channel and round into Pembrokeshire.
Global distribution
Recorded from Sweden and the western Baltic, the coasts of Denmark and German Bight, south to Portugal and Morocco.
Habitat
Lagoons and sheltered estuarine sites, where it inhabits a mud tube in muddy sediments.
Depth range
Low intertidal to shallow sublittoralIdentifying features
- Very small, less than 5 mm long.
- Three pairs of gills.
- 16 thoracic and 13-19 abdominal chaeta-bearing segments.
Additional information
No text entered
Listed by
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Annelida | Segmented worms e.g. ragworms, tubeworms, fanworms and spoon worms |
| Class | Polychaeta | Bristleworms, e.g. ragworms, scaleworms, paddleworms, fanworms, tubeworms and spoon worms |
| Order | Terebellida | |
| Family | Ampharetidae | |
| Genus | Alkmaria | |
| Authority | Horst, 1919 | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Moderate density | ||
| Male size range | 3-5 mm | ||
| Male size at maturity | |||
| Female size range | 3-5 mm | ||
| Female size at maturity | |||
| Growth form | Vermiform segmented | ||
| Growth rate | Data deficient | ||
| Body flexibility | High (greater than 45 degrees) | ||
| Mobility | Burrower | ||
| Characteristic feeding method | Surface deposit feeder | ||
| Diet/food source | Detritivore | ||
| Typically feeds on | Detritus | ||
| Sociability | Not relevant | ||
| Environmental position | Infaunal | ||
| Dependency | None. | ||
| Supports | Host Asymphylodora demeli | ||
| Is the species harmful? | No | ||
Biology information
Adults live within the sediment in durable mud tubes, the top of which protrude above the sediment surface. The tubes are two to three centimetres long and glued together by a rust-coloured paste. A large part of the tube is covered by large faecal pellets (Thorson, 1946). No information is available on adult growth rate, however, larval stages grow at approximately 0.15 mm/week (Cazaux, 1982).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Estuary, Isolated saline water (Lagoon), Ria or Voe |
| Biological zone preferences | Lower eulittoral, Sublittoral fringe |
| Substratum / habitat preferences | Mud, Muddy gravel, Muddy sand |
| Tidal strength preferences | Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Extremely sheltered, Sheltered, Ultra sheltered, Very sheltered |
| Salinity preferences | Low (<18 psu), Variable (18-40 psu) |
| Depth range | Low intertidal to shallow sublittoral |
| Other preferences | Apparently intolerant of long periods of emersion (Gilliland & sanderson, 2000). |
| Migration Pattern | Non-migratory or resident |
Habitat Information
Alkmaria romijni has been recorded from 27 sites around the UK (Gilliland & Sanderson, 2000; Thomas & Thorp, 1994). The majority of these are estuaries and the remainder lagoons. The species may be under-recorded due to it's small size. Alkmaria romijni is known from salinities of 5 to 48 ppt, but it's preferred range is thought to be 5 to 20 ppt (Gilliland & Sanderson, 2000).
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | No information |
| Fecundity (number of eggs) | 11-100 |
| Generation time | Insufficient information |
| Age at maturity | Insufficient information |
| Season | June - july |
| Life span | Insufficient information |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | Trochophore |
| Larval/juvenile development | Lecithotrophic, See additional information |
| Duration of larval stage | Not relevant |
| Larval dispersal potential | 10 -100 m |
| Larval settlement period | Not relevant |
Life history information
In Danish waters (Ringkøbing Fjord), Thorson (1946) noted that Alkmaria romijni was a protandrous hermaphodite, developing male gametes and then female eggs. All specimens contined ripe gametes in June and July but were smaller and empty in November. Development is not pelagic. The number of eggs per adult varied between 5 and 95 (an average of 57) ,and several speciments had 20 to 30 junveniles attached to the motuth of thier tubes (Thorson, 1946). Larval development lasts 3 months (Cazaux, 1982). Larvae reside within the tubes of the female for up to the first twelve days. They then become free-living on the surface of the sediment and develop their own tube at about 20 days (Cazaux, 1982).
Sensitivity review
Resilience and recovery rates
Alkmaria romijni is a small (3-5 mm when adult) polychaete that lives on the surface of the sediment. It is a protandrous hermaphrodite that broods its larvae within the tube of the adult. Fecundity is relatively low and larval development takes three months. There is no pelagic phase and development is benthic in the proximity of the adult. Juveniles become free-living and develop their own tubes after 20 days (Thorson, 1946; Cazaux, 1982). Nevertheless, Alkmaria romijni is considered an opportunistic species (Borja et al., 2000; Cardoso et al., 2004b, 2007; Teixeira et al., 2009) probably due to its small size, short life cycle and ability to reach high densities in favourable conditions such as organic enrichment.
Other opportunistic polychaetes also show benthic development, e.g. Pygospio. Shull (1997) demonstrated that Pygospio elagans (and other polychaetes) was able to colonize sediments by burrowing, and bed load transport in mobilized sediment. It is possible that the small size of Alkmaria romijni would facilitate bed load transport and it may be capable of swimming as both juvenile and adult, although no evidence was found. For example, experimental defaunation studies have shown an increase in Pygospio elegans, higher than background abundances within 2 months, reaching maximum abundance within 100 days (Van Colen et al. 2008). Following a period of anoxia in the Bay of Somme (north France) that removed cockles, Pygospio elegans increased rapidly but then decreased as cockle abundance recovered and sediments were disturbed by cockle movement (Desprez et al., 1992). Re-colonization of Pygospio elegans was observed in 2 weeks by Dittmann et al. (1999) following a one month long defaunation of the sediment. However, McLusky et al. (1983) found that Pygospio elegans were significantly depleted for >100 days after harvesting (surpassing the study monitoring timeline). Ferns et al. (2000) found that tractor towed cockle harvesting removed 83% of Pygospio elegans (initial density 1850 per m2). In muddy sand habitats, Pygospio elegans had not recovered their original abundance after 174 days (Ferns et al., 2000). These results are supported by work by Moore (1991) who also found that cockle dredging can result in reduced densities of some polychaete species, including Pygospio elegans. Rostron (1995) undertook experimental dredging of sandflats with a mechanical cockle dredger, including a site comprised of stable, poorly sorted fine sands with small pools and Arenicola marina casts with some algal growths. At this site, post-dredging, there was a decreased number of Pygospio elegans with no recovery to pre-dredging numbers after six months.
Alkmaria romijni was thought to be a lagoonal specialist (Arndt, 1989) but Gilliland & Sanderson (2000) concluded that it was probably a brackish water species based on its distribution in both lagoons and estuaries in the UK. It is also recorded in estuarine, lagoonal and other transitional water bodies in the German Bight, Denmark, the Baltic and Portugal, where is can reach high densities. Gilliland & Sanderson (2000) suggested that it was under-recorded in the UK due to its small size and the need for specialist identification. Therefore, it is not limited to lagoons and may be more widespread than current records suggest (authors comment).
Alkmaria romijni was reported in the Russian part of the Vistula Lagoon (south-east Baltic) after 1996, an area in which it had not been recorded previously in extensive studies in the first three decades of the twentieth century, the 1950s and 1960s, probably due to increases in eutrophication (Ezhova et al., 2005). However, no information on possible routes of colonization was suggested. Thomas & Thorp (1994) reproted considerable variation in the abundance of the mud infauna withn the Emsworth millpond complex (Chichester Harbour) probably due to fluctuations in slainity. They noted that Alkmaria romijni exhibted moderate abundance in 1982 and 1987 samples, disappeared from 1989 samples and returned at high abundance in 1991.
Resilience assessment. It is possible that Alkmaria romijni could recover from disturbance rapidly in areas it occurs and, using Pygospio as an example and the observations of Thomas & Thorpe (1994), probably with 1 to 2 years. However, where it occurs in isolated lagoons, and the population is removed or lost, then recovery would probably depend on random, unpredictable events, such as storms that transport sediment bearing the species to the affected location. Therefore, where the species experiences significant disturbance (e.g. resistance is 'Medium' or 'Low') then resilience is probably 'High'. Where the population is severely affected (e.g. resistance is 'None') and habitat recovery is also required then resilience is probably 'Medium' (2-10 years). Similarly, where the population is severely affected (e.g. resistance is 'None') and occurs in an isolated lagoonal location then resilience is probably 'Medium' (2-10 years). However, the confidence in the assessment is recorded as 'Low' due to the scarcity of direct evidence.
Hydrological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Temperature increase (local) [Show more]Temperature increase (local)Benchmark. A 5°C increase in temperature for one month, or 2°C for one year. Further detail EvidenceArndt (1989) suggested that Alkmaria romijni was a thermophilic species. Arndt (1989) reported an LT50 (the lethal temperature at which 50% of specimens die) after 24 hrs of 38.4°C at 5‰ 38.9°C at 10‰ and 40.45°C at 20‰, based on Nausch (1985, Fig. 3). Thermal tolerance increased with increasing salinity (Nausch, 1984; Arndt, 1989). Alkmaria romijni also tolerated low temperature conditions and had a freezing LT50 of 4.4 min at -10°C (and 10‰) when acclimated at 10°C or 4.7 min when acclimated at 5°C (Nausch, 1984; Arndt, 1989). In comparison, Arndt (1989) also suggested that Streblospio shrubsoli (a species that often coexists with, and competes with, Alkmaria romijni) as also thermophilic as their tolerances were very similar (Nausch, 1984) while Hediste diversicolor and Fabricia stellaris were more thermophobic. Sensitivity assessment. The above evidence suggests that Alkmaria romijni is thermophilic with a wide tolerance of temperatures, which coupled with a distribution from the North Sea and the Baltic to Morocco, suggests that it is resistant of a change in temperate of 2°C for a year in UK waters. Similarly, it lives on the surface of sediment, in the lower intertidal and shallow subtidal, so may be exposed to warm summers and cold winters throughout its range, and a change in 5°C for a month may result in stress. Mortality may result where a thermal discharge coincides with the warmest months of the year, or from extreme winter events but no direct evidence was available. Therefore, a resistance of ‘High’ is suggested. Hence, resilience is ‘High’ and the species is recorded as ‘Not sensitive’ at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Temperature decrease (local) [Show more]Temperature decrease (local)Benchmark. A 5°C decrease in temperature for one month, or 2°C for one year. Further detail EvidenceArndt (1989) suggested that Alkmaria romijni was a thermophilic species. Arndt (1989) reported an LT50 (the lethal temperature at which 50% of specimens die) after 24 hrs of 38.4°C at 5‰ 38.9°C at 10‰ and 40.45°C at 20‰, based on Nausch (1985, Fig. 3). Thermal tolerance increased with increasing salinity (Nausch, 1984; Arndt, 1989). Alkmaria romijni also tolerated low temperature conditions and had a freezing LT50 of 4.4 min at -10°C (and 10‰) when acclimated at 10°C or 4.7 min when acclimated at 5°C (Nausch, 1984; Arndt, 1989). In comparison, Arndt (1989) also suggested that Streblospio shrubsoli (a species that often coexists with, and competes with, Alkmaria romijni) as also thermophilic as their tolerances were very similar (Nausch, 1984) while Hediste diversicolor and Fabricia stellaris were more thermophobic. Sensitivity assessment. The above evidence suggests that Alkmaria romijni is thermophilic with a wide tolerance of temperatures, which coupled with a distribution from the North Sea and the Baltic to Morocco, suggests that it is resistant of a change in temperate of 2°C for a year in UK waters. Similarly, it lives on the surface of sediment, in the lower intertidal and shallow subtidal, so may be exposed to warm summers and cold winters throughout its range, and a change in 5°C for a month may result in stress. Mortality may result where a thermal discharge coincides with the warmest months of the year, or from extreme winter events but no direct evidence was available. Therefore, a resistance of ‘High’ is suggested. Hence, resilience is ‘High’ and the species is recorded as ‘Not sensitive’ at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Salinity increase (local) [Show more]Salinity increase (local)Benchmark. A increase in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidenceRuso et al. (2007) reported that changes in the community structure of soft sediment communities due to desalinization plant effluent in Alicante, Spain. In particular, in close vicinity to the effluent, where the salinity reached 39 psu, the community of polychaetes, crustaceans and molluscs was lost and replaced by one dominated by nematodes. Roberts et al. (2010b) suggested that hypersaline effluent dispersed quickly (within 10s of metres of the outfall) but was more of a concern at the seabed and in areas of low energy where widespread alternations in the community of soft sediments were observed. In several studies, echinoderms and ascidians were amongst the most sensitive groups examined (Roberts et al., 2010b). Alkmaria romijni has been recorded form salinities of 5 to 48 psu but it's preferred range is thought to be 5 to 20 ppt since most records and the highest abundances are recorded in the latter range (Gilliland & Sanderson, 2000). Sensitivity assessment. An increase in salinity from full to >40 psu is may result in a reduction in the abundance Alkmaria romijni of over a period of a year (the benchmark). However, no direct no direct evidence of the effects of hypersaline conditions or effluent on the species was found. Therefore, ‘No evidence’ was recorded. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Salinity decrease (local) [Show more]Salinity decrease (local)Benchmark. A decrease in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidenceAlkmaria romijni is considered to be a brackish water species Arndt (1989). It is known from salinities of 5 to 48 ppt, but it's preferred range is thought to be 5 to 20 ppt since most records and the highest abundances are recorded in the latter range (Gilliland & Sanderson, 2000). In the Ria de Avereiro (Portugal) Alkmaria romijni reached its highest abundances in areas of low salinity (18-30 or 5-18, Fig 3) close to freshwater input (Rodrigues et al., 2011). In the Arade river estuary (Portugal) (Silva et al., 2012), it was also found close to freshwater input but was ubiquitous throughout the estuary and not a good indicator of the influence of groundwater in the system. Resilience assessment. Alkmaria romijni has a wide salinity tolerance (Gilliland & Sanderson, 2000). Therefore, it would probably be resistant of a change from ‘full’ or ‘variable’ salinity to ‘reduced’, or from ‘reduced’ to ‘low’ salinity regimes for a year, and may even benefit and increase in abundance due to loss of competition, and a resistance of ‘High’ is recorded. Hence, resilience is ‘High’ and the species is recorded as ‘Not sensitive’ at the benchmark level. However, a change to freshwater conditions may result in loss of species. | HighHelp | HighHelp | Not sensitiveHelp |
Water flow (tidal current) changes (local) [Show more]Water flow (tidal current) changes (local)Benchmark. A change in peak mean spring bed flow velocity of between 0.1 m/s to 0.2 m/s for more than one year. Further detail EvidenceAlkmaria romijni is only recorded from muddy sediments in lagoons, estuaries and other transitional waters that are sheltered from wave action and with weak tidal streams (e.g. <0.5 m/s). A further decrease in water flow is unlikely. However, an increase in water flow may result in mobilization of the sediment surface and removal of the species from the sediment surface. It is likely to result in modification of the sediment over a period of a year to more sandy and coarse sediments that are less suitable for the species. Therefore, while a 0.1-0.2 m/s change is small, the resultant change in the sediment, removal of fines, and potentially a proportion of the species' population, suggests a resistance of 'Low'. Resilience is probably 'High' and sensitivity of 'Low' is recorded. | LowHelp | HighHelp | LowHelp |
Emergence regime changes [Show more]Emergence regime changesBenchmark. 1) A change in the time covered or not covered by the sea for a period of ≥1 year or 2) an increase in relative sea level or decrease in high water level for ≥1 year. Further detail EvidenceGilliland and Sanderson (2000) suggest that Alkmaria romijni is intolerant of long periods of emersion based on its preference for the lower littoral and shallow sublittoral. Therefore, a decrease in emergence (an increase in the time covered by the tide) would probably allow the species to colonize sediments further up thee shore and expand its range. However, an increase in emergence is likely to reduce the upper limit of the species on the shore and, hence, reduce its range. Therefore, a resistance of 'Low' is suggested. Resilience is probably 'High' and sensitivity of 'Low' is recorded. | LowHelp | HighHelp | LowHelp |
Wave exposure changes (local) [Show more]Wave exposure changes (local)Benchmark. A change in near shore significant wave height of >3% but <5% for more than one year. Further detail EvidenceAlkmaria romijni is only recorded from muddy sediments in lagoons, estuaries and other transitional waters that are sheltered to extremely sheltered from wave action and with weak tidal streams (e.g. <0.5 m/s). A further decrease in wave action is unlikely. However, an increase in wave action may result in mobilization of the sediment surface and removal of the species from the sediment surface. It is likely to result in modification of the sediment over a period of a year to more sandy and coarse sediments that are less suitable for the species. Although a 3-5% change in significant wave height is small, the resultant change in the sediment, removal of fines, and potentially a proportion of the species' population, suggests a resistance of 'Medium'. Resilience is probably 'High' and sensitivity of 'Low' is recorded. | MediumHelp | HighHelp | LowHelp |
Chemical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Transition elements & organo-metal contamination [Show more]Transition elements & organo-metal contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Hydrocarbon & PAH contamination [Show more]Hydrocarbon & PAH contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Synthetic compound contamination [Show more]Synthetic compound contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Radionuclide contamination [Show more]Radionuclide contaminationBenchmark. An increase in 10µGy/h above background levels. Further detail EvidenceNo evidence was found | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction of other substances [Show more]Introduction of other substancesBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
De-oxygenation [Show more]De-oxygenationBenchmark. Exposure to dissolved oxygen concentration of less than or equal to 2 mg/l for one week (a change from WFD poor status to bad status). Further detail EvidenceNausch (1984) reported an LT50 (50% mortality) after 50 hours in anoxic conditions (oxygen free water) at 5°C but 32 hrs at 10°C. The addition of hydrogen sulphide reduced the LT50 to 33.2 hrs and 17.2 hrs respectively (Nausch, 1984). Cardoso et al. (2004) noted that algal mats of Ulva (as Enteromorpha) intestinalis resulted in a slight decline in the abundance of Alkmaria romijni, and that algal mats reduced the redox potential of the sediment (a sign of increased anoxia) but suggested that the decline in abundance of the species was due to the algal mats' interference with feeding. In several studies, Alkmaria romijni is associated with organically enriched sediments and eutrophic conditions (Borja et al., 2000; Cardoso et al., 2004b, 2007; Teixeira et al., 2009; Rodrigues et al., 2011). Organic-rich sediments tend to be hypoxic, although the species lives at the sediment surface exposed to passing water flow. In the Ria de Aveiro (Portugal) Alkmaria romijni reached its highest abundances in areas with a redox potential of 47.7 mV (Rodrigues et al., 2011). Sensitivity assessment. Alkmaria romijni achieves high abundances in organic-rich sediments, and in areas subject to eutrophication, and is probably exposed to low oxygen levels in the sediment. Therefore, it is probably resistant of a short-term reduction in oxygen levels below 2 mg/l (see benchmark) and a resistance of 'High' is recorded. Hence, resilience is 'High' and the species is probably 'Not sensitive' at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Nutrient enrichment [Show more]Nutrient enrichmentBenchmark. Compliance with WFD criteria for good status. Further detail EvidenceAlkmaria romijni is associated with organically enriched sediments and eutrophic conditions (Borja et al., 2000; Cardoso et al., 2004b, 2007; Teixeira et al., 2009; Rodrigues et al., 2011). Rodrigues et al. (2011) noted that Alkmaria romijni reached high abundance in the assemblage associated with increased organic content, increased fines and reduced hydrodynamic characteristics. Cardoso et al. (2004) noted that algal mats of Ulva (as Enteromorpha) intestinalis resulted in a slight decline in the abundance of Alkmaria romijni, and that algal mats reduced the redox potential of the sediment (a sign of increased anoxia) but suggested that the decline in abundance of the species was due to the algal mats' interference with feeding. Borja et al. (2000) placed Alkmaria romijni in AMBI category III, "species tolerant of excess organic matter enrichment; that occur under normal conditions but their populations are stimulated by enrichment". Teixeira et al. (2009) noted that the changes in ecological quality and recovery in Mondego estuary were better represented by the AMBI when Alkmaria romijni was classified in AMBI category IV, "second order opportunistic species". Cardoso et al. (2007) noted that Alkmaria romijni and Capitella capitata were indicators of organically enriched habitats and reached their highest abundances in the eutrophic areas of the estuary. Cardoso et al. (2007) noted that both species decreased in abundance after management was put in place in the 1990s to reduce eutrophication of the Mondego estuary. Resilience assessment. Several studies suggest that Alkmaria romijni occurs and benefits from eutrophic conditions. Excessive growth of algal mats may reduce its abundance slightly (Cardoso et al., 2004). The benchmark is relatively protective and is not set at a level that would allow blooms of green algae on the sediment, hence, resistance is assessed as 'High' and resilience as 'High' (by default) so that the biotope is assessed as 'Not sensitive' at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Organic enrichment [Show more]Organic enrichmentBenchmark. A deposit of 100 gC/m2/yr. Further detail EvidenceAlkmaria romijni is associated with organically enriched sediments and eutrophic conditions (Borja et al., 2000; Cardoso et al., 2004b, 2007; Teixeira et al., 2009; Rodrigues et al., 2011). Rodrigues et al. (2011) noted that Alkmaria romijni reached high abundance in the assemblage associated with increased organic content, increased fines and reduced hydrodynamic characteristics. Borja et al. (2000) placed Alkmaria romijni in AMBI category III, "species tolerant of excess organic matter enrichment; that occur under normal conditions but their populations are stimulated by enrichment". Teixeira et al. (2009) noted that the changes in ecological quality and recovery in Mondego estuary were better represented by the AMBI when Alkmaria romijni was classified in AMBI category IV, "second order opportunistic species". Cardoso et al. (2007) noted that Alkmaria romijni and Capitella capitata were indicators of organically enriched habitats and reached their highest abundances in the eutrophic areas of the estuary. Cardoso et al. (2007) noted that both species decreased in abundance after management was put in place in the 1990s to reduce eutrophication of the Mondego estuary. Resilience assessment. Alkmaria romijni is associated with naturally organic-rich sediments, benefits as a result of organic enrichment, and is an indicator of organic enrichment. Therefore, a resistance of 'High' is recorded with a resilience of 'High' and the species is recorded as 'Not sensitive'. | HighHelp | HighHelp | Not sensitiveHelp |
Physical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Physical loss (to land or freshwater habitat) [Show more]Physical loss (to land or freshwater habitat)Benchmark. A permanent loss of existing saline habitat within the site. Further detail EvidenceAll marine habitats and benthic species are considered to have a resistance of ‘None’ to this pressure and to be unable to recover from a permanent loss of habitat (resilience is ‘Very low’). Sensitivity within the direct spatial footprint of this pressure is therefore ‘High’. Although no specific evidence is described, confidence in this assessment is ‘High’ due to the incontrovertible nature of this pressure. | NoneHelp | Very LowHelp | HighHelp |
Physical change (to another seabed type) [Show more]Physical change (to another seabed type)Benchmark. Permanent change from sedimentary or soft rock substrata to hard rock or artificial substrata or vice-versa. Further detail EvidenceThe species lives in sediment so a change to an artificial or rock substratum would result in the loss of its habitat. Based on the loss of species habitat (substratum), resistance is assessed as ‘None’, resilience is assessed as ‘Very low’ (as the change at the pressure benchmark is permanent), and sensitivity is assessed as ‘High’. Although no specific evidence is described, confidence in this assessment is ‘High’ due to the incontrovertible nature of this pressure. | NoneHelp | Very LowHelp | HighHelp |
Physical change (to another sediment type) [Show more]Physical change (to another sediment type)Benchmark. Permanent change in one Folk class (based on UK SeaMap simplified classification). Further detail EvidenceAlkmaria romijni is recorded from muddy sediments, including muddy sands and muddy gravels. A change in sediment type from to sands or coarse sediments would result in loss of suitable habitat for the species. Therefore, a resistance of 'None' is recorded. Resilience is assessed as ‘Very low’ (as the change at the pressure benchmark is permanent), and sensitivity is assessed as ‘High’. Although no specific evidence is described, confidence in this assessment is ‘High’ due to the incontrovertible nature of this pressure. | NoneHelp | Very LowHelp | HighHelp |
Habitat structure changes - removal of substratum (extraction) [Show more]Habitat structure changes - removal of substratum (extraction)Benchmark. The extraction of substratum to 30 cm (where substratum includes sediments and soft rock but excludes hard bedrock). Further detail EvidenceAlkmaria romijni lives at the sediment surface in a tube of only two to three centimetres in length. Removal of 30 cm of the sediment would remove the entire population in the affected area. Therefore, a resistance of 'None' is recorded. Recovery will depend on the recovery of the sediment itself and subsequent colonisation. Therefore a resilience of 'Medium' is recorded and sensitivity is assessed as 'Medium'. | NoneHelp | MediumHelp | MediumHelp |
Abrasion / disturbance of the surface of the substratum or seabed [Show more]Abrasion / disturbance of the surface of the substratum or seabedBenchmark. Damage to surface features (e.g. species and physical structures within the habitat). Further detail EvidenceAlkmaria romijni lives at the sediment surface in a tube of only two to three centimetres in length. It is soft-bodied but small (3-5 mm in length). Physical disturbance via abrasion to the sediment surface, or via penetrative gear or activities is likely to damage and kill a proportion of the population depending on the size of the footprint of the activity. Therefore, a resistance of 'Low' is suggested, although no direct evidence was found. The resilience is probably 'High' and a sensitivity of 'Low' is recorded. | LowHelp | HighHelp | LowHelp |
Penetration or disturbance of the substratum subsurface [Show more]Penetration or disturbance of the substratum subsurfaceBenchmark. Damage to sub-surface features (e.g. species and physical structures within the habitat). Further detail EvidenceAlkmaria romijni lives at the sediment surface in a tube of only two to three centimetres in length. It is soft-bodied but small (3-5 mm in length). Physical disturbance via abrasion to the sediment surface, or via penetrative gear or activities is likely to damage and kill a proportion of the population depending on the size of the footprint of the activity. Therefore, a resistance of 'Low' is suggested, although no direct evidence was found. The resilience is probably 'High' and a sensitivity of 'Low' is recorded. | LowHelp | HighHelp | LowHelp |
Changes in suspended solids (water clarity) [Show more]Changes in suspended solids (water clarity)Benchmark. A change in one rank on the WFD (Water Framework Directive) scale e.g. from clear to intermediate for one year. Further detail EvidenceAlkmaria romijni is found at the surface of muddy sediments in low energy environments with low water flow, sheltered from wave action. It is also considered an indicator of organically enriched sediments and occurs in eutrophic habitats and in estuaries where turbidity can be high (Cole et al., 1999). Therefore, an increase in suspended sediment at the benchmark level is unlikely to be detrimental. A decrease in turbidity might be detrimental, as Cardoso et al. (2007) reported that the density of Alkmaria romijni decreased after mitigation measures were implemented in the Mondego estuary to reduce eutrophication and hence turbidity and organic loads. However, a decrease in turbidity alone was not the cause of the decline but rather the reduction in the organic content of the sediment and competition from larger polychaetes (Cardoso et al., 2007). In naturally organic-rich sediments, and changes in suspended sediment loads is unlikely to be detrimental. Therefore, resistance is assessed as 'High', resilience as 'High' and sensitivity recorded as 'Not sensitive' at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Smothering and siltation rate changes (light) [Show more]Smothering and siltation rate changes (light)Benchmark. ‘Light’ deposition of up to 5 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceAlkmaria romijni lives at the sediment surface in a tube of only two to three centimetres in length. It is soft-bodied but small (3-5 mm in length). It is found in low energy environments (low water flow, sheltered from wave action) so any deposited sediment is likely to remain for many tidal cycles. No information on its ability to burrow was found. However, Maurer et al. (1986) noted that mucous tube feeders and labial palp deposit feeders (such as Alkmaria romijni) were the most susceptible to the killing effects of burial. Therefore, burial by 5 cm of fine deposit (the benchmark) is likely to result in at least some mortality and a resistance of 'Medium' is recorded, albeit with low confidence. Resilience is probably 'High' so that sensitivity is assessed as 'Low'. | MediumHelp | HighHelp | LowHelp |
Smothering and siltation rate changes (heavy) [Show more]Smothering and siltation rate changes (heavy)Benchmark. ‘Heavy’ deposition of up to 30 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceAlkmaria romijni lives at the sediment surface in a tube of only two to three centimetres in length. It is soft-bodied but small (3-5 mm in length). It is found in low energy environments (low water flow, sheltered from wave action) so any deposited sediment is likely to remain for many tidal cycles. No information on its ability to burrow was found. However, Maurer et al. (1986) noted that mucous tube feeders and labial palp deposit feeders (such as Alkmaria romijni) were the most susceptible to the killing effects of burial. Therefore, burial by 30 cm of fine deposit (the benchmark) is likely to result in at least significant mortality and a resistance of 'Low' is recorded, albeit with low confidence. Resilience is probably 'High' so that sensitivity is assessed as 'Low'. | LowHelp | HighHelp | LowHelp |
Litter [Show more]LitterBenchmark. The introduction of man-made objects able to cause physical harm (surface, water column, seafloor or strandline). Further detail EvidenceNot assessed | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Electromagnetic changes [Show more]Electromagnetic changesBenchmark. A local electric field of 1 V/m or a local magnetic field of 10 µT. Further detail EvidenceNo evidence was found | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Underwater noise changes [Show more]Underwater noise changesBenchmark. MSFD indicator levels (SEL or peak SPL) exceeded for 20% of days in a calendar year. Further detail EvidenceNot relevant | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Introduction of light or shading [Show more]Introduction of light or shadingBenchmark. A change in incident light via anthropogenic means. Further detail EvidenceNot relevant | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Barrier to species movement [Show more]Barrier to species movementBenchmark. A permanent or temporary barrier to species movement over ≥50% of water body width or a 10% change in tidal excursion. Further detail EvidenceNot relevant. This pressure is considered applicable to mobile species, e.g. fish and marine mammals rather than seabed habitats. Physical and hydrographic barriers may limit the dispersal of spores, larvae and other propagules. But propagule dispersal is not considered under the pressure definition and benchmarks, | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Death or injury by collision [Show more]Death or injury by collisionBenchmark. Injury or mortality from collisions of biota with both static or moving structures due to 0.1% of tidal volume on an average tide, passing through an artificial structure. Further detail EvidenceNot relevant’ to seabed habitats. NB. Collision by grounding vessels is addressed under ‘surface abrasion. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Visual disturbance [Show more]Visual disturbanceBenchmark. The daily duration of transient visual cues exceeds 10% of the period of site occupancy by the feature. Further detail EvidenceNot relevant | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Biological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Genetic modification & translocation of indigenous species [Show more]Genetic modification & translocation of indigenous speciesBenchmark. Translocation of indigenous species or the introduction of genetically modified or genetically different populations of indigenous species that may result in changes in the genetic structure of local populations, hybridization, or change in community structure. Further detail EvidenceNo evidence of translocation, breeding or species hybridization was found. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction or spread of invasive non-indigenous species [Show more]Introduction or spread of invasive non-indigenous speciesBenchmark. The introduction of one or more invasive non-indigenous species (INIS). Further detail EvidenceThomas & Thorpe (1994) recorded Alkmaria romijni, Nematostella vectensis and other benthic infauna in the vicinty of aggregations of the non-native polychaete Ficopomatus enigmaticus, in the Emsworth millpond complex, Chichester Harbour. However, the variation in abundance of Alkmaria romijni in their 10-year study was not explained by the presense of aggregations of Ficopomatus enigmaticus. No evidence was found to suggest a positive or negative interaction between non-indigenous invasive species and Alkmaria romijni. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction of microbial pathogens [Show more]Introduction of microbial pathogensBenchmark. The introduction of relevant microbial pathogens or metazoan disease vectors to an area where they are currently not present (e.g. Martelia refringens and Bonamia, Avian influenza virus, viral Haemorrhagic Septicaemia virus). Further detail EvidenceNo evidence of microbial pathogens was found. Alkmaria romijni was reported to host the trematode parasite Asymphylodora demeli (Margolis, 1971) but no information on its effect, if any, at the population level was found. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Removal of target species [Show more]Removal of target speciesBenchmark. Removal of species targeted by fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidenceNot relevant. This species is not subject to a targetted commercial or recreational fishery. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Removal of non-target species [Show more]Removal of non-target speciesBenchmark. Removal of features or incidental non-targeted catch (by-catch) through targeted fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidenceNo evidence was found on the effects, if any, of commercial or recreational fishing on this species. However, it occurs in estuaries and transitional water bodies where it may be exposed to physical disturbance from a range of activities including, but not limited to, trawling, dredging, trampling and vehicular access, bait digging etc. The sensitivity to such activities is addressed under the 'abrasion' and 'penetration' pressure above. Alkmaria romijni can reach high abundances in estuaries, lagoons and transitional waters, but no biological interactions are known. It has been reported to compete with Streblospio shrubsoli where they coexist (Arndt, 1989) but no evidence on the biological effects of its removal from the ecosystem was found. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Importance review
Policy/legislation
| Designation | Support |
|---|---|
| Wildlife & Countryside Act | Schedule 5, section 9 |
| Species of principal importance (Wales) | Yes |
| Features of Conservation Importance (England & Wales) | Yes |
Status
| National (GB) importance | Nationally scarce | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | Native |
| Origin | Not relevant |
| Date Arrived | Not relevant |
Importance information
No text entered
Bibliography
Arndt, E.A., 1973. Ecophysiological and adaptational problems confronting animals living in brackish water. Oikos, Suppl. 15, 239-245.
Arndt, E.A., 1989. Ecological, physiological and historical aspects of brackish water fauna distribution. In Proceedings of the 23rd European Marine Biology Symposium, Swansea, 5-9 September 1988. Reproduction, Genetics and Distribution of Marine Organisms, (ed. J.S. Ryland & P.A. Tyler), pp. 327-338. Denmark: Olsen & Olsen.
Bamber, R.N., Batten, S.D. & Bridgwater, N.D., 1991. The brackish ponds at Killingholme, Humberside, UK. Aquatic Conservation: Marine and Freshwater Ecosystems, 1 (2), 173-181.
Bamber, R.N., Batten, S.D., Sheader, M. & Bridgwater, N.D., 1992. On the ecology of brackish water lagoons in Great Britain. Aquatic Conservation: Marine and Freshwater Ecosystems, 2 (1), 65-94.
Barnes, R.S.K., 1994. The brackish-water fauna of northwestern Europe. Cambridge: Cambridge University Press.
Borja, A., Franco, J. & Perez, V., 2000. A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Marine Pollution Bulletin, 40 (12), 1100-1114.
Cardoso, P.G., Bankovic, M., Raffaelli, D. & Pardal, M.A., 2007. Polychaete assemblages as indicators of habitat recovery in a temperate estuary under eutrophication. Estuarine, Coastal and Shelf Science, 71 (1-2), 301-308.
Cardoso, P.G., Pardal, M.A., Raffaelli, D., Baeta, A. & Marques, J.C., 2004b. Macroinvertebrate response to different species of macroalgal mats and the role of disturbance history. Journal of Experimental Marine Biology and Ecology, 308 (2), 207-220.
Cazaux, C., 1982. Developpement larvaire de l'ampharetidae lagunaire Alkmaria romijni Horst, 1919. Cahiers de Biologie Marine, 23, 143-157.
Cole, S., Codling, I.D., Parr, W. & Zabel, T., 1999. Guidelines for managing water quality impacts within UK European Marine sites. Natura 2000 report prepared for the UK Marine SACs Project. 441 pp., Swindon: Water Research Council on behalf of EN, SNH, CCW, JNCC, SAMS and EHS. [UK Marine SACs Project.]. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/water_quality.pdf
Desprez, M.H., Rybarczyk, H., Wilson, J.G., Ducrotoy, J.P., Sueur, F., Olivesi, R. & Elkaim, B., 1992. Biological impact of eutrophication in the Bay of Somme and the induction and impact of anoxia. Netherlands Journal of Sea Research, 30, 149-159.
Dittmann, S., Günther, C-P. & Schleier, U., 1999. Recolonization of tidal flats after disturbance. In The Wadden Sea ecosystem: stability, properties and mechanisms (ed. S. Dittmann), pp.175-192. Berlin: Springer-Verlag.
Ezhova, E., Zmudzinski, L. & Maciejewska, K., 2005. Long-term trends in the macrozoobenthos of the Vistula Lagoon, southeastern Baltic Sea. Species composition and biomass distribution. Bulletin of the Sea Fisheries Institute, 1 (164), 55-73.
Ferns, P.N., Rostron, D.M. & Siman, H.Y., 2000. Effects of mechanical cockle harvesting on intertidal communities. Journal of Applied Ecology, 37, 464-474.
Gilliland, P.M., & Sanderson, W.G., 2000. Re-evaluation of marine benthic species of nature conservation importance: a new perspective on certain 'lagoonal specialists' with particular emphasis on Alkmaria romijni Horst (Polychaeta: Ampharitidae). Aquatic Conservation: Marine and Freshwater Ecosystems, 10(1), 1-12.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Margolis, L., 1971. Polychaetes as Intermediate Hosts of Helminth Parasites of Vertebrates: A Review. Journal of the Fisheries Research Board of Canada, 28 (10), 1385-1392.
Maurer, D., Keck, R.T., Tinsman, J.C., Leatham, W.A., Wethe, C., Lord, C. & Church, T.M., 1986. Vertical migration and mortality of marine benthos in dredged material: a synthesis. Internationale Revue der Gesamten Hydrobiologie, 71, 49-63. DOI https://doi.org/10.1002/iroh.19860710106
Moore, J., 1991. Studies on the Impact of Hydraulic Cockle Dredging on Intertidal Sediment Flat Communities. A report to the Nature Conservancy Council from the Field Studies Council Research Centre, Pembroke, Wales, FSC/RC/4/91.
Nausch, M., 1984. The distribution of Streblospio shrubsoli, Alkmaria romijni and Fabricia sabella and their resistance to temperature, oxygen deficiency and hydrogen sulphide. Limnologica, 15, 497-501.
Roberts, D.A., Johnston, E.L. & Knott, N.A., 2010b. Impacts of desalination plant discharges on the marine environment: A critical review of published studies. Water Research, 44 (18), 5117-5128.
Rodrigues, A.M., Quintino, V., Sampaio, L., Freitas, R. & Neves, R., 2011. Benthic biodiversity patterns in Ria de Aveiro, Western Portugal: Environmental-biological relationships. Estuarine, Coastal and Shelf Science, 95 (2–3), 338-348.
Rostron, D., 1995. The effects of mechanised cockle harvesting on the invertebrate fauna of Llanrhidian sands. In Burry Inlet and Loughor Estuary Symposium, pp. 111-117.
Ruso, Y.D.P., la Ossa Carretero, J.A.D., Casalduero, F.G. & Lizaso, J.L.S., 2007. Spatial and temporal changes in infaunal communities inhabiting soft-bottoms affected by brine discharge. Marine environmental research, 64 (4), 492-503.
Shull, D.H., 1997. Mechanisms of infaunal polychaete dispersal and colonisation in an intertidal sandflat. Journal of Marine Research, 55, 153-179.
Silva, A.C.F., Tavares, P., Shapouri, M., Stigter, T.Y., Monteiro, J.P., Machado, M., Cancela da Fonseca, L. & Ribeiro, L., 2012. Estuarine biodiversity as an indicator of groundwater discharge. Estuarine Coastal and Shelf Science, 97, 38-43.
Teixeira, H., Neto, J.M., Patrício, J., Veríssimo, H., Pinto, R., Salas, F. & Marques, J.C., 2009. Quality assessment of benthic macroinvertebrates under the scope of WFD using BAT, the Benthic Assessment Tool. Marine Pollution Bulletin, 58 (10), 1477-1486.
Thomas, N.S. & Thorp, C.H., 1994. Cyclical changes in the fauna associated with tube aggregates of Ficopomatus enigmaticus (Fauvel). Memoires du Museum National d'Histoire Naturelle, 162, 575-584.
Thorson, G., 1946. Reproduction and larval development of Danish marine bottom invertebrates, with special reference to the planktonic larvae in the Sound (Øresund). Meddelelser fra Kommissionen for Danmarks Fiskeri- Og Havundersögelser, Serie: Plankton, 4, 1-523.
Van Colen, C., Montserrat, F., Vincx, M., Herman, P.M., Ysebaert, T. & Degraer, S., 2008. Macrobenthic recovery from hypoxia in an estuarine tidal mudflat. Marine Ecology-Progress Series, 372, 31-42.
Datasets
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2024. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2024-05-21
West Wales Biodiversity Information Centre, 2017. WTSWW Data: All Taxa (West Wales). Occurrence dataset: https://doi.org/10.15468/gaakk2 accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 09/02/2017
- Lagoon