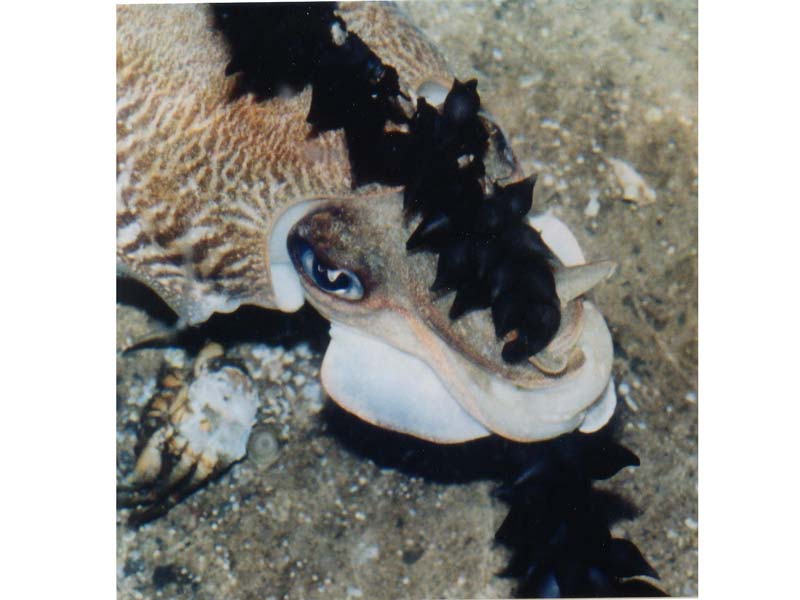
Common cuttlefish (Sepia officinalis)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Eliza Gibson-Hall & Emily Wilson | Refereed by | This information is not refereed |
| Authority | Linnaeus, 1758 | ||
| Other common names | - | Synonyms | - |
Summary
Description
The common cuttlefish Sepia officinalis is a relatively broad and somewhat flattened cephalopod, appearing oval in cross section. It has a mantle length of up to 45 cm. Paired fins run from behind the head to the tip of the body. Sepia officinalis has eight arms and two elongated tentacles. These arms are specialised for grasping prey after it has been seized by the tentacles. The tentacles are cylindrical, muscular structures which can be quickly elongated. They end in a tentacular club, with five to six rows of suckers, that is specially adapted for prey capture. Males have adapted one of the arms for the purpose of transporting sperm into the female’s buccal cavity, located in her mouth. Individuals are capable of very rapid colour change, especially when threatened. They may also take the colour or patterning of its background.
Recorded distribution in Britain and Ireland
Recorded along the south coast and west coast of England and Wales and around the Channel Islands and the Isle of Man. Sporadic records exist along the east coast of Britain and the west to the north coast of Scotland, including the Hebrides. Some records from the north-east and south-west of Ireland.
Global distribution
Recorded from the eastern Atlantic; from the Baltic and North Sea to South Africa and in the Mediterranean.
Habitat
Found on sandy and muddy substrata, in the shallow sublittoral and offshore to 200 m, but typically to 100 m depth. It is a demersal species and prefers moderately warm, shallow coastal waters as well as continental shelves.
Depth range
0-200 mIdentifying features
- They have eight arms and two tentacles which both end in a tentacular club with 5-6 rows of suckers.
- Cuttlebone anteriorly and posteriorly rounded, with parallel sides.
- Colour - very variable; may be black-brown, striped or mottled on the dorsal surface, paler to white on the ventral surface.
Additional information
Males are larger than females, and slightly more frequent (Dunn, 1999). Sepia elegans is smaller, has two rows of suckers on arms and has an acute lobe on its dorsal mantle margin. Sepia officinalis has been observed to undergo a ‘sleep-like state’. Observed behaviour included a quiescent state, changes in body colouration, twitching of arms, and rapid eye movement, which could be an indication of REM sleep (Frank et al., 2012). This state occurs periodically during periods of inactivity and can last on average 135 seconds (Frank et al., 2012). Cuttlefish is also said to have one of the largest brain-to-body rations of any invertebrate.
Listed by
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Mollusca | Snails, slugs, mussels, cockles, clams & squid |
| Class | Cephalopoda | Cuttlefish, nautilus, octopus & squid |
| Order | Sepiida | Cuttlefish |
| Family | Sepiidae | |
| Genus | Sepia | |
| Authority | Linnaeus, 1758 | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Moderate density | ||
| Male size range | 8-45 cm | ||
| Male size at maturity | 14-24 cm | ||
| Female size range | 12-29 cm | ||
| Female size at maturity | 15-22 cm | ||
| Growth form | No information | ||
| Growth rate | 1.4-3.3cm/month | ||
| Body flexibility | Low (10-45 degrees) | ||
| Mobility | Swimmer | ||
| Characteristic feeding method | Predator | ||
| Diet/food source | Heterotroph | ||
| Typically feeds on | Brachyuran crabs and demersal fish (gobiids and syngnathids), with minor amounts of amphipods and little cannibalism. | ||
| Sociability | Solitary | ||
| Environmental position | Demersal | ||
| Dependency | Independent. | ||
| Supports | Host Doridicola longicauda (Claus 1860) | ||
| Is the species harmful? | Yes Saliva contains cephalotoxin which induces paralysis and immobilizes prey. | ||
Biology information
Size. Size is measured as dorsal mantle length. The sizes of breeding individuals range from eight to 45 cm in males and from 12 to 29 cm in females (Goff & Daguzan, 1991). The largest individual to be recorded reached 60 cm in length (Compton & Wiley, 2011). Due to a variation in size amongst individuals, juveniles are usually defined as non-mature adults (Guerra, 2006) (see size at maturity).
Growth. The growth rate of juveniles is generally faster than adults. In juveniles, growth rates also depend on the month of hatching. Juveniles hatched in early summer, June, had a growth rate of around 1.2 mm/day. In comparison, juveniles born in late summer, August, had a reduced growth rate of around 0.5 mm/day. This is thought to be due to a difference in water temperature during embryonic development and post-hatching (Challier et al., 2002). Nutrition and the availability of food is also important for the initial growth rate of juveniles. Cultured juveniles that were starved during the first twenty days of rearing displayed a negative growth rate and ultimately died (Koueta & Boucaud-Camou, 2001).
Adults have a more linear growth rate (Challier et al., 2005) that almost ceases during winter migration to colder offshore waters (Dunn, 1999). The growth rate of Sepia officinalis decreases continuously with an increase in size (Domingues et al., 2001). A maximum lifespan of two years is common with the exception of some individuals reaching four years of age in culture (Bettencourt & Guerra, 1999). In areas of higher temperatures, such as the Portuguese coast, the time taken to reach sexual maturity is far less compared to English, colder, waters. This results in a reduced lifespan of one year (Gras et al., 2016).
Camouflage ability. One of the primary defence mechanisms against potential predators is to camouflage and stay hidden. Sepia officinalis, amongst other cephalopod species, are able to undergo countershading. This behaviour means that if an individual is rotated 90 degrees their chromophores expand and contract accordingly to make sure the underside of the body is now the colour of the upper half and vice versa. This allows the cuttlefish to remain camouflaged and prevents it from being conspicuous (Ferguson et al., 1994). Cuttlefish are able to scale the intensity of their body patterning depending on the light intensity surrounding them. This is thought to allow them to conserve energy (Buresch et al., 2015). In extremely low light conditions Sepia officinalis does not appear to camouflage to the substratum (Buresch et al., 2015).
Mobility and sociability. Sepia officinalis is a mobile species. Buoyancy is maintained by the species’ cuttlebone. The internal cuttlebone is incredibly porous and is used by the species to adjust buoyancy by increasing and decreasing liquid flow into the bone (Hastie et al., 2009). Sepia officinalis are not very sociable and do not form shoals in the wild. In culture, they will tolerate each other unless they are under periods of extreme food deprivation (Guerra, 2006). Juveniles tend to show a high tolerance for other individuals compared to adults. After four months post-hatching a feeding hierarchy is clearly visible in culture (Hanlon & Messenger, 1996).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Estuary, Open coast, Ria or Voe, Sea loch or Sea lough |
| Biological zone preferences | Lower circalittoral, Lower infralittoral, Sublittoral fringe, Upper circalittoral, Upper infralittoral |
| Substratum / habitat preferences | Coarse clean sand, Fine clean sand, Mud, Muddy sand, Sandy mud |
| Tidal strength preferences | No information |
| Wave exposure preferences | No information |
| Salinity preferences | Variable (18-40 psu) |
| Depth range | 0-200 m |
| Other preferences | No text entered |
| Migration Pattern | Seasonal (environment), Seasonal (reproduction) |
Habitat Information
The common cuttlefish occurs in both warm and temperate environments and migrates to and from each environment depending on the stage in the life cycle (Boletzky, 1983). They occur predominantly on sandy-muddy bottoms from the coastline to approximately 200 m. The most common occurrence is at 100 m. Below 200 m, the ambient pressure of the water is enough to implode the inner shell (Ward & Boletzky, 1984). The shells of newly hatched individuals can implode between 50 and 100 m, meaning hatchlings are found in shallow coastal waters (Guerra, 2006). Juveniles occur in coastal habitats with a preference for sandy substratum (for hiding) and seagrass beds. Seagrass beds are highly productive areas and serve as a protective environment providing shelter for juveniles (Jackson et al., 2001). Seagrass also reduces currents and promotes sediment deposition providing the correct environment for juveniles (Camou, 2001). In the central Cíes islands, egg abundance was highest in the most sheltered zones in depths of 8 to 13 m (Guerra & Castro, 1998; Guerra et al., 2016b).
Sepia officinalis is the most northerly-distributed cuttlefish. Climate change models have predicted that the species may be able to expand its range up to the warming arctic waters. Temperatures would need to be above 7°C as lower temperatures reduce the metabolic processes of the species (Xavier et al., 2016). If Sepia officinalis were able to reach into the Arctic Circle and down into the western Atlantic, it could reach the east coast of America by 2300 (Xavier et al., 2016). The short maturity time and the constant cycle of generations mean that cephalopods, compared to other groups, can adjust faster to a change in environmental conditions (Xavier et al., 2016). Finally, if the species can reach Iceland, Greenland or the Faroe Islands, they will find seagrass beds and/or kelp that are a perfect habitat for spawning (Xavier et al., 2016).
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | Semelparous or monotely |
| Fecundity (number of eggs) | 100-1,000 |
| Generation time | 1-2 years |
| Age at maturity | 13-16 months |
| Season | March - July |
| Life span | 1-2 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | Not relevant |
| Larval/juvenile development | See additional information |
| Duration of larval stage | Not relevant |
| Larval dispersal potential | No information |
| Larval settlement period | Not relevant |
Life history information
Lifespan. If food availability is restricted in early life this may result in delayed maturation and an extended lifespan (Boletzky, 1974). Mortality in females occurs after spawning, which can take several weeks to a few months (Guerra, 2006). Biannual life cycles have been observed frequently. In the English Channel, hatchlings born from July to September grew rapidly. The juveniles migrate from the inshore nursery grounds to overwintering in deeper waters. They return inshore as soon as they exhibit the first signs of sexual maturity (Guerra, 2006).
Size at maturity. Maturity in males usually occurs by September at around thirteen months old (Guerra, 2006). Maturity is based on observation of both testicle size and the position of spermatophores (Gras et al., 2016). Female maturation begins slightly later and tends to take longer (Guerra, 2006). Maturity in females is characterized by the size of the oocyte and the development of the nidamental glands (Gras et al., 2016). There appears to be an annual variation in the size at sexual maturity. In 2011, the size at which sexual maturity was reached for both sexes, in the English Channel, was lower than in 2010. For example, sexually mature males were 14 cm in 2011 compared to 16 cm in 2010 (Gras et al., 2016). There is also a variation between group I breeders and group II breeders. Group I breeders, which spawn during the first year of life, reach sexual maturity at around 14.5 cm (14 cm males, 15 cm females). However, Group II breeders, reproduce during their second year of life and are able to grow for longer and reach maturity at ca 23 cm (24 cm males, 22 cm females) (Gauvrit et al., 1997). Temperature also appears to affect the age at which juveniles reach sexual maturity. Individuals reared at 20°C became sexually mature at seven months and were 14 cm in length. In contrast, those raised at 10°C remained immature at seven months and measured only 5 cm long (Richard, 1996 as cited in Bloor et al., 2013).
Fecundity. In the English Channel, females lay, on average, only 200-550 eggs depending on the size of the female (Boletzky, 1987). The potential fecundity of sexually mature females in the Aegean Sea is 3,700 to 8,000 oocytes (Laptikhovsky et al., 2003). Despite this potential, the average number of eggs released from spawning females was 1000-3000 prior to death (Bloor et al., 2013) in the English Channel. It has therefore been suggested that Sepia officinalis females release an equivalent of 50% of their potential fecundity. In culture, fecundity decreased with each generation resulting in the 7th generation being almost infertile. Forsythe et al. (1994) suggested that this could be due to the cultures having a longer lifespan and, therefore, putting more energy into growth rather than reproduction. Bacteria also play an important role in the reproductive cycle. Symbiotic bacteria are responsible for the colour change in the accessory nidamental glands. This colour change from white to a bright red/orange signifies reproductive readiness. This is thought to play a role in mating and could also impact the production of fertile eggs (Forsythe et al., 1994). In the same culture, Forsythe et al. (1994) observed a lack of pigmentation on the gonads as well as a series of gonad infections. The lack of important bacteria in the culture could also have an impact on the fertility of individuals.
Migration. Inshore migration is governed by reproduction with individuals travelling from several to hundreds of nautical miles (Mangold, 1966). Migration for spawning occurs when an individual reaches maturity (Rodhouse & Nigmatullin, 1996). Mature males and large females of Sepia officinalis are the first to migrate inshore to spawning grounds in March and April with spawning occurring from March to July. Migratory patterns have been observed in the English Channel, mostly by fisheries’ landing data (Bloor et al., 2013). Hatchlings then emerge during the summer period and undergo rapid growth before beginning their autumn migration offshore to overwintering grounds in the deep central waters (Bloor et al., 2013). The purpose of migrating back inshore to spawn is due to a preferred habitat for eggs and juveniles (Bloor et al., 2013). In the English Channel, offshore migration occurs in winter and is influenced primarily by a reduction in water temperature as well as the reduction in daylight hours (Guerra, 2006). After spawning occurs adults return back into deeper, offshore areas around October in the UK (Dunn, 1999, Royer et al., 2006). The total distance migrated in each season may exceed 100 km (Du Sel & Daguzan, 1997). Sexually mature individuals do not migrate immediately to an area. Some tagged individuals have been observed to travel 23-35 km along the coast during spawning in 14 days (Bloor et al., 2013b).
Mating. Mating occurs in deeper water. Mate choice occurs in female Sepia officinalis and appears to be a preference, not for dominance, but for the absence of zebra banding. This might be because zebra branding infers aggression in the male (Boal, 1997). Males carry up to 1400 spermatophores and females 150-4000 eggs depending on their size. Spermatophores are placed in the female’s buccal membrane located above the mouth (Guerra, 2006). A single pair can mate several times and males have been observed to guard their mate post-insemination (Hanlon et al., 1999). Females are polyandrous and can accept and store sperm from multiple males, and can control which sperm to fertilize her eggs (Naud et al., 2005). This is referred to as the ‘cryptic female choice hypothesis’ (Eberhard, 1996). More food was consumed by sexually mature females than non-sexually mature females and mature males suggesting a higher demand (Castro & Guerra, 1999).
Spawning. The mantle of female Sepia officinalis increases by approximately 10% during the first months of spawning. Females are ‘intermittent terminal spawners’ as they lay eggs in separate batches (Rocha et al, 2001). For many females, the first phase of laying is slow and linear, ending in an exponential phase of vast egg laying (Boletzky, 1987). Spawning occurs in shallow water (see sexual migration) and peaks at water temperatures of 13-15°C (Guerra, 2006). In the English Channel, the spawning season extends from February to July but, in warmer summers, spawning has been observed in August (Dunn, 1999, Wang et al., 2003). Therefore, temperature is thought to be the main regulating factor on the length of spawning (Goff and Daguzan, 1991). If water temperatures remain above 10°C during winter, spawning can occur year-round e.g. in the Ria de Vigo (Guerra & Castro, 1998; Bloor et al., 2013). Eggs are rarely laid at depths greater than 30-40 m (Boletzky, 1983). The eggs are attached in grape-like clusters to various seaweeds, seagrass, and sessile animals such as tubeworms or dead structures (Bloor et al., 2013). Eggs can also be attached to previously laid cuttlefish eggs. Attaching eggs to seaweed and seagrass can elevate the eggs and reduce the risk of predation. If conditions are not ‘suitable’, females can choose how many eggs are laid to reduce the mortality of all her offspring (Bloor et al., 2013). In Morbihan Bay, south Brittany, 18 – 40 million Sepia officinalis eggs are laid on cuttle-traps from March to September. These traps are emptied every two days by the fishermen but are then stacked on pontoons until further use. This results in the mortality of many of the eggs due to their damage and removal from the water (Blanc & Daguzan, 1998).
Embryonic development. Eggs are flask-shaped, coated in ink from the mother and approximately 3-4 cm in length (Boletzky, 1983). As well as acting as a protective barrier, the egg capsule can also protect against environmental stress and microbial infection (Catarina et al., 2017). Bacteria also live inside the egg and provide antifouling and antimicrobial properties alongside a yolk supplying nutrients. Sepia officinalis eggs are one of the largest among cephalopods (Challier et al., 2005). Egg development can last from 1 to 5 months depending on the water temperature (Catarina et al., 2017). At 12°C development took 5 months in culture with development ceasing at temperatures below 9°C. However, once temperatures were increased embryogenesis restarted (Bouchaud & Daguzan, 1990, Challier et al., 2004). Eggs cultured at 15°C appeared to use 41% of the egg yolk for growth and 10% for respiration and excretion. Eggs cultured at 24°C, however, used only 15% of the egg yolk for growth and used 52% for respiration and excretion. Therefore, Sepia officinalis hatched at 24°C were almost half the size of hatchlings grown in 15°C. All the eggs were from the same mother and were around the same size of 23 cm (Bouchaud, 1991). The optimal temperature for embryonic development is 15-18°C (Bouchaud, 1991). Maternal health and nutritional history are also known to influence the size and success of offspring (Steer et al., 2004, cited in Bloor et al., 2013). Catarina et al. (2017) reported that Sepia officinalis' eggs are predated by; “the snail Bolinus brandaris, the crab Cancer pagurus, the hermit crab Dardanus arrosor, the lobster Homarus gammarus, the invasive blue crab Callinectes sapidus, the shrimp Squilla mantis, the sea urchins Echinus melo, Cidaris sp. and Paracentrotus lividus, and the starfish Astropecten aranciacus”. The egg capsules become thinner and lose their ink making them more transparent towards the end of embryonic development. In this last stage, the eggs are most vulnerable to predation (Catarina et al., 2017). Oxygen also becomes limiting towards the end of embryonic development as both oxygen consumption and ammonia production are at their highest within the egg capsule (Lesser, 2010).
Hatching. In the English Channel, hatching is usually the highest in July and August but may begin as early as May (Challier et al., 2002). The hatchlings are similar to adults in morphology and basic behaviour but only 7-8 mm in length (Boletzky, 1983). No parental care has been observed. Hatchlings are developed enough to actively feed within hours of hatching. They have the ability to adapt if the food supply is limited by reducing their growth rate (Boletzky, 1983). Juveniles will stay in breeding grounds until 60 - 120 days old, whereupon they join adults in offshore, winter grounds, (Blanc & Daguzan, 1999, Challier et al., 2002). The newly hatched cuttlefish will survive off of the inner yolk reserve from the egg for up to the first 305 days or until the reserve ‘runs out’ (Blanc et al., 1998). The first ten days post-hatching are the most vulnerable for the hatchlings and is the period of highest mortality (Bloor et al., 2013). Hatchlings show an immediate behavioural response to visual or odour cues. This is thought to suggest that embryos can detect stimuli during development, before hatching (Romagny et al., 2012). Hatchlings also show habituation to certain repeated visual stimuli (O’Brien et al., 2017). Embryos hatched in culture undertake sand digging during the first six days. This is presumed to be the initial and preferred predator response for small, newly hatched, Sepia officinalis. In order to bury in the sand, the cuttlefish squirts a series of jets of water from a ventral funnel, allowing it to ‘wiggle’ into the sand. (Boletzky, 1987; Mather, 1986 as cited in Poirier et al., 2004).
Juveniles. Juveniles are able to produce a uniform body pattern during the first few months post-hatching (O’Brien et al., 2017) and are able to detect water movements which are key anti-predatory behaviours. In juveniles, 30-60% of food intake is estimated to be used for growth (Koueta & Bouchaud, 2001). Juveniles are able to catch shrimps with ease in complete darkness suggesting an adapted ability to detect prey (Blanc et al., 1998). In the English Channel, they begin to migrate to deeper water in late August (Wang et al., 2003). After migrating back to the in-shore spawning grounds in spring many juveniles will show some signs of sexual maturity. However, they will usually not be fully mature until 13 months old for males and 14-16 for females (Dunn, 1999). In south Brittany, juveniles born in mid-March to June may become sexually mature as early as November (males) or December (females). This is again thought to be due to the higher temperatures, decreasing the time taken to develop and resulting in a life cycle of only one year (Gauvrit et al., 1998). Most individuals spawn after their second migration offshore-inshore and are known as the second group of breeders. These individuals are larger as they have had longer to grow before reaching sexual maturity (Gauvrit et al., 1998). Although mortality usually occurs post-reproduction in second-year breeders, some individuals have been reported to even live to three years old (Mattacola et al., 1984).
Population dynamics. Sepia officinalis contributes to around 5-10% of landings to local fisheries in the Mediterranean Sea (Salman et al., 1997, Turan & Yalioglu, 2010). Sepia officinalis stocks are comprised of two overlapping annual cohorts of migrating adults and newly hatched juveniles. The English Channel stock has a very high recruitment of 44 – 79 million, which is higher than many other fished cephalopods in the region (Royer et al., 2006). Landing data has shown that older animals are subject to the highest levels of mortality due to fishing. This is thought to be due to fishermen actively targeting larger individuals especially during the inshore migration period (Royer et al., 2006). If adults are targeted more than juveniles later in the year this is beneficial to recruitment and ensures the size of the stock survives. However, if adults are targeted before spawning this could have a dire effect on the following year’s recruitment and population (Royer et al., 2006). Global landings of cephalopods have increased from 0.5 million tonnes in 1958 to 4 million tonnes in 2008 (FAO 1964 & 2012). It is expected that landings will increase as fishing pressure subsequently increases following the decline of other marine stocks (Bloor et al., 2013). Bloor & Jackson (2014) reported a change during the Marine Biological Association's scientific trawls from dominance by Sepia officinalis to an increase in the less commercially important Sepia elegans. Further analysis is yet to be undertaken but this may suggest a shift in the dominant species.
There is evidence for low levels of genetic variation between populations from the Bay of Biscay, the English Channel and the southern North Sea (Wolfram et al., 2006). However, they cannot be regarded as freely reproducing populations as there is still a lack of evidence of migration from the Bay of Biscay into the English Channel (Wolfram et al., 2006). There is a significant correlation between geographical separation and an increase in genetic distance between the waters of the Atlantic and Mediterranean (Perez-Losada et al., 1999). The isolation is said to have occurred due to sea level changes during glacial maxima with a subsequent reconnection. This has led to subpopulations with restricted gene flow. The lack of genetic exchange is also a result of females fixing their eggs to the seafloor and a lack of a larval phase (Perez-Losada et al., 2002). Genetic, morphological and chemical differences have been seen between populations in Turkish coastal waters. The chemical structure of the cuttlebone has shown at least four geographical populations of Sepia officinalis in Turkish waters (Turan & Yaglioglu, 2010). These genetic differences may be due to the limited dispersal ability of Sepia officinalis alongside the lack of a planktonic larval stage (Turan & Yaglioglu, 2010).
Sensitivity review
Resilience and recovery rates
Sepia officinalis has a short lifespan of between one to two years with mortality occurring shortly after spawning. This means that the recruitment of the population relies heavily on the restocking of individuals to replace the mass mortality of adults after spawning (Hastie et al., 2009, Challier et al., 2006). This short, fast-growing, lifespan means that each year’s stock is almost entirely made up of one population, or two in the English Channel (Royer et al., 2006). Juveniles reach sexual maturity relatively early either in the following summer or the summer after depending on the temperature of the water (Bloor et al., 2013). Cultured Sepia officinalis are able to reproduce easily and are able to lay large eggs with a high chance of survival even under artificial conditions (Blanc et al., 1999). There is usually only one breeding season for females before mortality occurs. Fecundity per individual is usually only 200-500 eggs and is size-dependent (Boletzky, 1987). This is only thought to be 50% of their potential fecundity but the smaller number of eggs means the female can increase the energy yield of the internal yolk, allowing embryos to grow larger and stronger (Boletzky, 1987).
Sepia officinalis is also known as an ‘intermittent terminal spawner’. This means they do not lay all of their eggs in a single place. Instead, they move up the coastline and spawn in particularly favourable conditions and environments. This increases the survival chance of the offspring (Rocha et al., 2001). Eggs are also surrounded by ink from the female which acts as a protective barrier (Catarina et al., 2017). Maternal stress during spawning is likely to affect their offspring’s phenotypes. Stress is associated with a reduction in offspring fitness and resistance to pressures such as disease (O’Brien et al., 2017). Despite the lack of parental care post-hatching, the hatchlings are developed enough to begin actively feeding almost as soon as hatching is complete (Boletzky, 1983). The juveniles also show anti-predatory behaviour within the first few months including camouflage and burying (O’Brien et al., 2017). There is, therefore, a significant lack of overlap between generations. This leaves the species vulnerable to annual fluctuations in recruitment and stock size (Koueta et al., 2000).
Complex recruitment. The annual variation in Sepia officinalis recruitment in the English Channel is estimated at 44-79 million individuals (Royer et al., 2006). Successful recruitment requires sufficient breeding rates with a high survival of hatchlings (Boyle, 1990). There are also annual variations in recruitment and size at which maturity is reached (Gras et al., 2016). Females are polyandrous and therefore can accept sperm from multiple males, picking the sperm of choice to fertilize her offspring. This is beneficial as it means reproduction is not limited to a single, preferred male fertilizing all the females, and increases the chance of fertilization (Bloor et al., 2013).
Environmental conditions. Sepia officinalis is not a vulnerable species to environmental conditions. On the contrary, the species tends to have a great ability to adapt to a range of different conditions (Sobrino et al., 2002). They do, however, require particular spawning habitats to ensure successful embryonic development and hatching (Bloor et al., 2013). Sepia officinalis are efficient feeders and have tentacles which can reach prey within 15 milliseconds (Hanlon & Messenger, 1996). They are opportunistic feeders, eating a wide range of species allowing them to adapt to a variety of different prey (Jorge & Sobra, 2004). Sepia officinalis feeds on prey belonging to four different groups; polychaete, cephalopods, crustaceans and bony fish. However, the variety of prey consumed decreases with an increase in size (Castro & Guerra, 1999). A group of starved cuttlefish were able to survive for up to 21 days post-hatching (Koueta & Boucaud-Camou, 2001). However, Sepia officinalis does not appear to have the ability to store food as energy in its tissues. If there is a restriction of food during early development it means sexual maturity will be reached later. This means they require a regular food supply in order to survive and more importantly, reach sexual maturity and reproduce (Gras et al., 2016). Sepia officinalis is also a very mobile species and therefore is able to move to another area which has more suitable conditions (Guerra, 2006).
Genetic differentiation. The restricted exchange in genetic information may lead to population-specific variations in chemical structures and morphology (Carvalho and Hauser, 1994; Turan et al., 2006; Turan & Yaglioglu, 2010). Such variations in morphology may affect the adaptability of Sepia officinalis to different environments. In areas of high water flow, individuals with bigger tentacular clubs and suckers have a better ability to cling onto rocks and avoid damage (Ozsoy et al., 1996). The differences in morphology may limit different populations to certain areas restricting recruitment from neighbouring populations (Turan & Yaglioglu, 2010). There appears to be a cross-over in populations around the English Channel and the southern North Sea. However, there is a clear genetic difference between the Atlantic and Mediterranean populations (Wolfram et al., 2006). In terms of recruitment, it appears that the lack of genetic similarity is due to a lack of movement of populations. This lack of external recruitment also occurs due to the lack of a larval pelagic stage (Pérez-Losada et al., 2002).
Resilience. Recruitment to and recovery of populations is likely to be rapid depending on whether juveniles or adults are removed from the population and at what life stage this occurs. Therefore, recovery from any loss of the population (i.e. a reduction in the extent or abundance, i.e. resistance is 'Medium') may take up to three to five years for a replacement of population by a recovering generation. Hence, a resilience of 'Medium' will be recorded. However, where the population is severely reduced in abundance or extent (i.e. resistance is 'None') a resilience of ‘Very low’ will be recorded.
Hydrological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Temperature increase (local) [Show more]Temperature increase (local)Benchmark. A 5°C increase in temperature for one month, or 2°C for one year. Further detail EvidenceCephalopods have a poikilothermic metabolism that rises or falls directly with temperature (Forsythe, 1993; cited in Bloor et al., 2013). For example, an increase of 6°C results in a doubling of individual feeding rate (Pascual, 1978). Cuttlefish in the English Channel have lower mitochondrial capabilities compared to subtropical Adriatic species but have larger hearts, which improve their energetic efficiencies. This allows them to extend their thermal tolerance (Oellermann et al., 2012). It has been estimated that the temperature limits of Sepia officinalis are 10 and 30°C (Guerra, 2006). In the Ria Formosa, Portugal, water temperature reaches 27°C in the summer Lagoon system. The observed number of Sepia officinalis infers they can survive high water temperatures (Domingues et al., 2002). Water temperature reached 30°C during the hottest day but no mortality was recorded. However, as temperatures rose in the Cíes Islands, Spain, the probability of finding eggs decreased. Instead, individuals in the Mediterranean prefer a spawning temperature of 12.5 – 14.75°C, similar to that of peak spawning in the English Channel (Guerra et al., 2016b). Embryonic development increases rapidly with an increase in temperature. At 17°C cultures, embryonic development took 56 days, resulting in a hatchling length of 8.4 mm mantle length (ML). In contrast, an increase in temperature to 20°C resulted in embryonic development taking just 40 days with hatchlings measuring a shorter 7.9 mm ML (Dickel et al., 1997). Warmer waters are, therefore, associated with a shorter incubation time (Sykes et al., 2006 b). However, a reduction in size could make the hatchlings more vulnerable to predation, reducing recruitment (Bouchaud, 1991). Sensitivity assessment. Sepia officinalis appear to be able to withstand high temperatures. However, for an extended period of time, it could affect their behaviour, development and recruitment. Therefore, resistance is scored as ‘Medium’. Resilience is likely to be ‘Medium’ and sensitivity is, therefore, ‘Medium’. | MediumHelp | MediumHelp | MediumHelp |
Temperature decrease (local) [Show more]Temperature decrease (local)Benchmark. A 5°C decrease in temperature for one month, or 2°C for one year. Further detail EvidenceIn the English Channel, water temperatures are known to get as low as 4°C in coastal areas (CEFAS, 2010, cited in Bloor et al., 2013). A decrease in water temperature was directly correlated with the offshore migration of Sepia officinalis during winter months (Wang et al., 2003). In the Adriatic Sea, where temperatures rarely fall beneath 12°C, no seasonal migration has been observed (Wolfram et al., 2006). Individuals also reach sexual maturity later in colder waters (Boletzky, 1983), with a preferred spawning temperature of 9.5 – 20°C (Paulij et al., 1990). It has been estimated that the temperature limits of Sepia officinalis are 10 and 30°C. Below 10°C individuals do not appear to feed and remain inactive until ultimate mortality (Guerra, 2006). Individuals hatched in lower temperatures also have less inner yolk, presumably due to the increase in embryonic development time. This means once hatched the juveniles are under more pressure to gather food (Guerra, 2006). Embryos ceased development when cultured at 9°C. However, when the temperature was raised development continued, which suggested that eggs could almost go into a ‘hibernation’ period under lower temperatures. It is not known how long this period can last for before mortality (Bouchaud & Daguzan, 1990; Challier et al., 2004). Oxygen demand for embryos is also increased at lower temperatures making embryos in colder water more susceptible to hypoxic conditions within the egg sac (Woods, 1999; cited in Lesser, 2013). Sensitivity assessment: Sepia officinalis appear to be able to survive at low temperatures but suffer mortality after prolonged exposure to 10°C or lower. However, as temperatures decrease in coastal waters individuals may be expected to retreat to deeper waters, with the development of eggs and recruitment potentially affected. Therefore, resistance is scored as ‘Medium’. Resilience is likely to be ‘Medium’ and sensitivity is, therefore ‘Medium’. | MediumHelp | MediumHelp | MediumHelp |
Salinity increase (local) [Show more]Salinity increase (local)Benchmark. A increase in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidenceSepia officinalis inhabits oceanic waters that are of full salinity and is unlikely to experience an increase of salinity. In addition, this species is mobile and is, therefore, unlikely to remain in areas of salinity higher than 37 psu (e.g. from hypersaline effluents). Therefore, it is probably ‘Not Sensitive’ at this benchmark level (i.e. resistance and resilience are ‘High’) | HighHelp | HighHelp | Not sensitiveHelp |
Salinity decrease (local) [Show more]Salinity decrease (local)Benchmark. A decrease in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidenceAdults have been observed to tolerate brackish water, especially during inshore spawning periods. Adult individuals have been observed in the Ria de Vigo (NW Spain) where salinity in the inlet can range from 20-35 PSU. Despite this, eggs have been laid in this inlet (Guerra & Castro, 1998). Nevertheless, the preferred spawning salinity is reported to be 28 or greater (Paulij et al., 1990). Adult Sepia officinalis are able to survive in salinities of 18 PSU but only if acclimatised slowly (Guerra & Castro, 1988). However, adults are sufficiently mobile and are able to move to a new area if conditions are unfavourable. Juveniles are thought to have a higher plasticity to changes in salinity allowing them to live in more sheltered lagoons with higher freshwater influxes (Paulij et al., 1990). However, normal embryonic development requires salinities above 24 ppt (Paulij et al., 1990). Below 22.4 PSU malformed embryos were observed and the development rate of embryos was reduced even at 28.7 PSU. This is due to an increase in osmotic stress and a resultant reduction in available energy reserves (Paulij et al., 1990). The highest percentage of healthy hatchlings was found at salinities of 29.8 PSU (Paulij et al., 1990). Therefore, in culture, the optimum working salinity range is 27 – 35 PSU (Sykes et al., 2006(b)). Sensitivity assessment. It appears that adults and juvenile cuttlefish are able to survive in areas with a decrease from full salinity of ca. 10 PSU. However, embryos appear to be more sensitive to a decrease in salinity and adults can reduce their spawning or have a decreased growth rate. The short life span of this species, an annual reproductive cycle and its high fecundity means that recovery should take a year. Therefore, resistance to this pressure is ‘Medium’. Resilience is probably ‘Medium' and sensitivity is, therefore ‘Medium‘. | MediumHelp | MediumHelp | MediumHelp |
Water flow (tidal current) changes (local) [Show more]Water flow (tidal current) changes (local)Benchmark. A change in peak mean spring bed flow velocity of between 0.1 m/s to 0.2 m/s for more than one year. Further detail EvidenceYoung hatchlings of only a month old responded to strong tidal currents by burrowing in the sand. Komak et al. (2005) reported a lack of migration and movement of juveniles under strong, turbulent, conditions. Therefore, it is important that juveniles have the ability to attach or bury deep enough in the substratum to withstand strong water movement (Boletzky, 1983). Adults were observed to align their bodies parallel to water flow when placed in a flow of 0.08 m/s even when this opposed the pattern of the background (Shohet et al., 2006). Sensitivity assessment. Sepia officinalis does not appear to be negatively affected by a change in tidal current, therefore, this species is probably ‘Not Sensitive’ with a ‘High’ resistance and a 'High' resilience. | HighHelp | HighHelp | Not sensitiveHelp |
Emergence regime changes [Show more]Emergence regime changesBenchmark. 1) A change in the time covered or not covered by the sea for a period of ≥1 year or 2) an increase in relative sea level or decrease in high water level for ≥1 year. Further detail EvidenceSepia officinalis are sublittoral and so will not be affected by an increase in emergence at the benchmark level. The species is fast moving and will avoid emergence. Sepia officinalis embryos encased in eggs can be transported for culture for up to 8.5 h in damp or dry conditions or in seawater. Observations of a reduction in oxygen consumption are thought to protect the developing embryos from the damp and dry conditions. The survival rate of transported eggs was 83 – 86% for both eggs kept in water or in dry conditions (Jones et al., 2009). Sensitivity assessment. Therefore, resistance is probably ‘High’, resilience is ‘High’ (by default) and this species is assessed as ‘Not Sensitive’ at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Wave exposure changes (local) [Show more]Wave exposure changes (local)Benchmark. A change in near shore significant wave height of >3% but <5% for more than one year. Further detail EvidenceIn rough weather, cuttlebones are often found washed up on beaches. This may mean that some of the population are destroyed when wave exposure increases or it could cause the already, naturally, deceased to wash up. Despite these observations, the species is sufficiently mobile to be able to move to deeper water if conditions are unfavourable. Therefore, resistance is probably ‘High’, resilience ‘High’ (by default) and this species is assessed as ‘Not Sensitive’ at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Chemical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Transition elements & organo-metal contamination [Show more]Transition elements & organo-metal contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail Evidence‘Not Assessed’ | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Hydrocarbon & PAH contamination [Show more]Hydrocarbon & PAH contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail Evidence‘Not Assessed’ | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Synthetic compound contamination [Show more]Synthetic compound contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail Evidence‘Not Assessed’ | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Radionuclide contamination [Show more]Radionuclide contaminationBenchmark. An increase in 10µGy/h above background levels. Further detail EvidenceNo evidence | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction of other substances [Show more]Introduction of other substancesBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail Evidence‘Not Assessed’ | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
De-oxygenation [Show more]De-oxygenationBenchmark. Exposure to dissolved oxygen concentration of less than or equal to 2 mg/l for one week (a change from WFD poor status to bad status). Further detail EvidenceSepia officinalis survived 60 mins in tanks at 50% of normal dissolved oxygen, 16.28 mg/l (Capaz et al., 2017). However, Sepia officinalis reduced their oxygen consumption to 37% and increased their ventilation rate to 85%. Metabolic rate subsequently decreased and a minor increase in anaerobic respiration was shown by an accumulation of octopine (lactic acid) (Capaz et al., 2017). Surprisingly, there was no recorded stress response of the animal (no increase in heat-shock protein 70). Capaz et al. (2017) suggested a lack of stress associated with a decrease in dissolved oxygen. Oxygen extraction from ambient water at 17°C is thought to be 70% (Melzner et al., 2006). Cephalopods also appear to have a more highly closed pressurised vascular system compared to other molluscs allowing them to distribute the O2 taken up by the gills (Häfker, 2012). Sepia officinalis will be vulnerable to areas of hypoxia when migrating inshore to sheltered bays and estuaries in order to spawn (Guerra, 2006). Boletzky (1981) suggested that the oxygen saturation of the surrounding water must be close to full saturation for efficient development and successful sexual maturity. Sepia officinalis embryos are encased in a protective egg-case which protects it from infection, certain predation and acts as a barrier for gas diffusion (Cronin & Seymour, 2000, Catarina et al., 2017). During the end of embryonic development, oxygen consumption significantly increases, relying heavily on a good supply of dissolved oxygen from the surrounding waters at levels as close to saturation as possible (Cronin & Seymour, 2000). Sepia officinalis embryos encased in eggs can be transported for culture for up to 8.5 hr in damp or dry conditions or in seawater. A reduction in oxygen consumption is thought to protect the developing embryos from the damp and dry conditions. The survival rate of transported eggs was 83 – 86% for both eggs kept in water or in dry conditions (Jones et al., 2009). It must be reminded that this is a mobile species and, therefore, after hatching the likelihood of remaining in an area of low oxygen is unlikely. Sensitivity assessment. Sepia officinalis appears to be adapted to survive in a dissolved oxygen concentration of 16.28 mg/l by reducing their oxygen consumption or moving away from the area. This, however, is higher than the benchmark of 2 mg/l for this pressure. De-oxygenation is thought to affect the development of embryos. Therefore resistance is assessed as ‘Medium’ due to the potential effect on recruitment. Resilience is probably ‘Medium’ and sensitivity is, therefore ‘Medium’. | MediumHelp | MediumHelp | MediumHelp |
Nutrient enrichment [Show more]Nutrient enrichmentBenchmark. Compliance with WFD criteria for good status. Further detail EvidenceRefers to the increased levels of nitrogen, phosphorus and silicon in the marine environment. Nutrient enrichment from pollution and other sources can lead to an increase in algal blooms. In turn, this can lead to periods of deoxygenation (see deoxygenation pressure). No evidence on the effect of nutrient enrichment specific to this species was found. However, the species is considered to be 'Not sensitive' at the pressure benchmark that assumes compliance with WFD criteria for good status. | HighHelp | HighHelp | Not sensitiveHelp |
Organic enrichment [Show more]Organic enrichmentBenchmark. A deposit of 100 gC/m2/yr. Further detail EvidenceOrganic enrichment encourages the productivity of suspension and deposit-feeding detrivores and allows species to colonize the affected area to take advantage of the enhanced food. Suspended organic material in the water column will eventually settle on the seabed. However, this has the potential to deoxidize the benthic environment due to the decomposition of the material causing hypoxia (Riera et al., 2013). Sepia officinalis is mostly a mobile demersal species and will probably avoid areas if hypoxia. Therefore, this species at this benchmark is likely to be ‘Not Sensitive’ (see de-oxygenation). | HighHelp | HighHelp | Not sensitiveHelp |
Physical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Physical loss (to land or freshwater habitat) [Show more]Physical loss (to land or freshwater habitat)Benchmark. A permanent loss of existing saline habitat within the site. Further detail EvidenceAdult Sepia officinalis are able to migrate from offshore habitats to inshore, but can also choose where they would like to lay their eggs. This means that if an environment is not suitable, the individual will continue moving in order to find a more suitable location to live and/or spawn (Bloor et al., 2013). Eggs, hatchlings and juveniles are more vulnerable to the permanent loss of a habitat due to their preference for a particular environment (Camou, 2001). If a structure was to suddenly be placed or an area of land claimed then this would result in the immediate mortality of any small individuals/eggs in that area. Without shallow, sandy, areas recruitment potential may be reduced due to limitation for space and resources (see habitat removal). Sensitivity assessment. Adults are unlikely to be directly affected by the physical loss of an area as they have highly mobile abilities. Juveniles, hatchlings and eggs are more vulnerable to immediate mortality and a lack of available habitat to survive. Therefore, the resistance is assessed as 'Medium' but with 'low' confidence. Resilience is probably 'Medium' and sensitivity is, therefore, 'Medium'. | MediumHelp | MediumHelp | MediumHelp |
Physical change (to another seabed type) [Show more]Physical change (to another seabed type)Benchmark. Permanent change from sedimentary or soft rock substrata to hard rock or artificial substrata or vice-versa. Further detail EvidenceHatchlings bury themselves in response to threats (e.g. predators) due to their lack of ability to camouflage or flee (O’Brien et al., 2017). Therefore, newly hatched embryos and developing juveniles require sandy substrata to avoid predation. Duration of digging also takes far longer in gravel compared to fine sand and some cuttlefish even refuse to bury into gravel making them vulnerable to predation (Mather, 1986). Juveniles and eggs are usually placed in sheltered areas such as seagrass beds. This reduces their vulnerability to predation and the impact of tides and water surges (Jackson et al., 2001). Without such shelters mortality is likely to occur, reducing recruitment. A lack of an appropriate environment for offspring may result in the female choosing not to lay any eggs at all reducing potential recruitment for the following population (Bloor et al., 2013). However, adults are highly mobile and, therefore, unlikely to be affected by a change in substratum since it can relocate to more favourable conditions. Sensitivity assessment. Resistance is probably ‘Medium’, however, confidence is ‘Low’ due to lack of evidence of mortality. Due to the lack of overlap in generations, survival of hatchlings is vital for replenishment and recruitment, therefore, resilience is considered ‘Medium’ and sensitivity is assessed as ‘Medium’. | MediumHelp | MediumHelp | MediumHelp |
Physical change (to another sediment type) [Show more]Physical change (to another sediment type)Benchmark. Permanent change in one Folk class (based on UK SeaMap simplified classification). Further detail EvidenceAdult Sepia officinalis will not be affected by a change in sediment class due to the species being highly mobile. However, juveniles rely heavily on sandy bottoms in order to shelter and hide from predation (see juveniles). A change in sediment class has not, however, shown to result directly in an increase in mortality rates. Sensitivity assessment. Hatchlings and juveniles are probably the most vulnerable to this pressure, as a change to muddy, coarse or hard substratum may increase their vulnerability to predation, therefore, resistance is assessed as ‘Medium’ but with ‘Low’ confidence due to a lack of evidence. Resilience is probably ‘Medium’ and sensitivity is, therefore, ‘Medium’. | MediumHelp | MediumHelp | MediumHelp |
Habitat structure changes - removal of substratum (extraction) [Show more]Habitat structure changes - removal of substratum (extraction)Benchmark. The extraction of substratum to 30 cm (where substratum includes sediments and soft rock but excludes hard bedrock). Further detail EvidenceThe act of physically removing the substratum e.g. by dredging may affect the cuttlefish (see physical disturbance). The ability of juveniles to attach themselves onto hard substratum is very important in ensuring they can withstand strong water movements (Boletzky, 1983). Juveniles and hatchlings also need to burrow into sandy substratum in order to avoid predation (Boletzky, 1983). There is also the risk that hatchlings and eggs buried or attached to the substratum will be removed along with the substratum resulting in mortality. Sensitivity assessment. The loss of habitat is unlikely to affect mobile adults but may damage or remove eggs, hatchlings and/or juveniles, reducing recruitment and further spawning due to a lack of suitable substratum. Therefore, resistance is likely to be ‘Low’ but with ‘low’ confidence due to a lack of evidence. Resilience is probably ‘Medium’ and sensitivity is, therefore, ‘Medium’. | LowHelp | MediumHelp | MediumHelp |
Abrasion / disturbance of the surface of the substratum or seabed [Show more]Abrasion / disturbance of the surface of the substratum or seabedBenchmark. Damage to surface features (e.g. species and physical structures within the habitat). Further detail EvidenceCuttlefish have a soft, unarmoured body making them vulnerable to damage and injury (Hanlon & Messenger, 1991; Cooke & Tonkins, 2015). The cuttlebone is able to repair itself and maintains buoyancy so that cuttlefish can withstand a certain amount of internal bone injury (Boletzky & Overath, 1991). Individuals observed after mating and after entanglement in nets typically have soft tissue damage that suggests susceptibility to abrasive damage (Guerra et al., 2016b). In a southern otter trawl, 77% of the landed Sepia officinalis were discarded suggesting high levels of damage occurring from these trawls (Guerra et al., 2016b). CEFAS has recorded discards of cuttlefish in the English Channel from 6% to 23% of total catch since 2002 (ICES, 2012). Enever et al. (2007) showed that Sepia officinalis was the second most discarded species under the top ten most discarded English Channel species by beam trawlers. Sepia officinalis from a large beam trawler operating in the offshore waters of south-west UK were examined during a single fishing trip for signs of damage and survival (Revill, 2011). Analysis showed that 16% of these small cuttlefish (<15 cm) died later after their injuries from capture, 68% died whilst in the trawl or on the deck, and only 16% survived capture and deck sorting and were expected to survive discarding (Revill, 2011). Individuals with mantle lesions and trauma are also susceptible to developing bacterial sepsis (Sherrill et al., 2000). However, the short life-span of this species, its annual reproduction and its high fecundity means that population recovery from such effects should occur after a year. Abrasion, due to trawling and dredging, may also remove biogenic structures such as seaweed and seagrass which are locations of attached eggs. If eggs have already been laid they are likely to be destroyed and the area is left as an unfavourable spawning ground (Bloor & Jackson, 2014). Sensitivity assessment. Adults are able to move out of the way of a lot of danger but are at risk of being caught and/or damaged by fishing equipment. However, juveniles and eggs are more vulnerable due to their reduced ability to move out of the way. Therefore, resistance is assessed as ‘Low’ but with 'low' confidence. Resilience is probably ‘Medium’ and sensitivity is assessed as ‘Medium’. | LowHelp | MediumHelp | MediumHelp |
Penetration or disturbance of the substratum subsurface [Show more]Penetration or disturbance of the substratum subsurfaceBenchmark. Damage to sub-surface features (e.g. species and physical structures within the habitat). Further detail EvidenceSepia officinalis is a mobile species and therefore is not likely to be affected by penetration by fishing gear, dredges or other activities. However, the primary defensive behaviour of juveniles or smaller, weaker, individuals is to bury into the sandy sea-floor. Penetration could therefore directly injury or damage the buried individual, affecting the recruitment rates for the population. Therefore, based on the evidence above (abrasion), resistance is assessed as ‘Low’ but with 'low' confidence. Resilience is probably ‘Medium’ and sensitivity is assessed as ‘Medium’. | LowHelp | MediumHelp | MediumHelp |
Changes in suspended solids (water clarity) [Show more]Changes in suspended solids (water clarity)Benchmark. A change in one rank on the WFD (Water Framework Directive) scale e.g. from clear to intermediate for one year. Further detail EvidenceSepia officinalis may benefit from reduced water clarity by acting as an external camouflage so that the species can preserve more energy. This may help reduce the chances of predation especially on the eggs of Sepia officinalis (Catarina et al., 2017). But, suspended solids may inhibit the species from effectively feeding by removing visual cues (Mäthger et al., 2006) as well as interfere with mating rituals. However, Sepia officinalis is a highly intelligent animal and is able to use other cues, such as chemical or audial, in order to track down prey and other individuals (Samson et al., 2014). Juveniles are able to catch shrimps with ease in complete darkness (Blanc et al., 1998). Furthermore, Jozet-Alves et al. (2013) suggested that Sepia officinalis may have an episodic-memory allowing the cuttlefish to remember key prey hotspots and feeding locations. Sensitivity assessment. Resistance is likely to be ‘High’ but with ‘low’ confidence. Resilience is probably ‘High’ and sensitivity is, therefore, assessed as ‘Not sensitive’. | HighHelp | HighHelp | Not sensitiveHelp |
Smothering and siltation rate changes (light) [Show more]Smothering and siltation rate changes (light)Benchmark. ‘Light’ deposition of up to 5 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceThe species is highly mobile and therefore unlikely to be affected by smothering since it can relocate to more favourable conditions. Juveniles are also able to move and dig into and out of small and large grain sand (Boletzky, 1983). Eggs are usually laid on floating or suspended structures in the water column and therefore are unlikely to be affected by light sediment deposition (Bloor et al., 2013). Sensitivity assessment. Resistance is, therefore, likely to be ‘High’. Resilience is ‘High’ by default and sensitivity is therefore assessed as ‘Not sensitive’. | HighHelp | HighHelp | Not sensitiveHelp |
Smothering and siltation rate changes (heavy) [Show more]Smothering and siltation rate changes (heavy)Benchmark. ‘Heavy’ deposition of up to 30 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceThe species is highly mobile and therefore unlikely to be affected by smothering since it can relocate to more favourable conditions. Juveniles are also able to move and dig into and out of deposited sediment (Boletzky, 1983). The duration of the digging and the juvenile’s ability to dig depends on the sediment (Mather, 1986). If there is a heavy deposition of coarse sand then juveniles should be able to dig straight out. As this benchmark is for the deposition of fine material and not hard gravel like substrata, there should be relatively low, if any, impact on the juveniles (Mather, 1986). Eggs are usually laid on floating or suspended structures in the water column and therefore are unlikely to be affected by heavy sediment (Bloor et al., 2013). Sensitivity assessment. Resistance is therefore likely to be ‘High’. Resilience is probably ‘High’ and sensitivity is, therefore ‘Not sensitive’. | HighHelp | HighHelp | Not sensitiveHelp |
Litter [Show more]LitterBenchmark. The introduction of man-made objects able to cause physical harm (surface, water column, seafloor or strandline). Further detail Evidence'Not assessed'. However, it is noted that man-made objects that have been left or dumped into the sea, such as ropes, are a popular spot for Sepia officinalis females to attach their eggs. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Electromagnetic changes [Show more]Electromagnetic changesBenchmark. A local electric field of 1 V/m or a local magnetic field of 10 µT. Further detail Evidence'No evidence' | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Underwater noise changes [Show more]Underwater noise changesBenchmark. MSFD indicator levels (SEL or peak SPL) exceeded for 20% of days in a calendar year. Further detail EvidenceThe statocyst is considered the primary sound detection organ in cephalopods. Alongside peripheral hairs and epidermal hairs, Sepia officinalis is able to detect local water movement by detecting vibrations (Hanlon & Messenger, 1996; Samson et al., 2014). The statocyst is also responsive for equilibrium and movement in the water column (Solé et al., 2017). In aquarium facilities in Portaferry, Northern Ireland, 30 individual Sepia officinalis were subject to 210 seconds of playback of engine noise recorded from a car ferry (165 Db). The noise appeared to affect the colour changing behaviour and movement of the cuttlefish. Individuals changed colour more frequently and increased locomotion compared to a control group with a playback of ambient reef noises. These behaviours suggested an increase in stress level (Kunc et al., 2014). As cephalopods use complex movements and behaviour during courtship, mating and camouflage, noise pollution may increase predation risk and decrease the chances of reproduction (Kunc et al., 2014). Noise was also reported to increase occurrences of jetting and inking (Hanlon & Messenger, 1996). The greatest intensity response e.g. inking, was found at the highest sound levels (100-300 Hz) (Samson et al., 2014) and above 140 Db. Sepia officinalis was able to habituate (get used to) the noise when identical sounds were played close together (Samson et al., 2014). This observation suggested that Sepia officinalis would not show a long-term response to sound. Individuals exposed to levels of sound from 139-142 dB centred at 315-400 Hz revealed injuries to the statocyst. Immediately after exposure to the sound, spherical holes were found on the base of the hair cells and in the plasma membrane of the statocyst. After 48 h of sound exposure damage occurred on the internal epithelium and some hair cells were either partially or totally ejected from the sensory epithelium. This could result in permanent physical damage but due to the dissections needed for this study the long-term effects are still unknown (Solé et al., 2017). If the species is disturbed by noise it should swim away when it senses any vibrations. The viability of the species may be reduced if feeding or breeding periods are disrupted. Sensitivity assessment. Sepia officinalis are able to undergo habituation to the sound but may change their behaviour, including mating behaviour. Internal damage has been caused by specific noise levels, meaning, the resistance to this pressure is probably ‘Medium’ albeit with ‘low’ confidence. Hence, resilience is probably ‘Medium’, and sensitivity has been assessed as ‘Medium’. | MediumHelp | MediumHelp | MediumHelp |
Introduction of light or shading [Show more]Introduction of light or shadingBenchmark. A change in incident light via anthropogenic means. Further detail EvidenceSepia officinalis appears to be sensitive to light. Under a bright light in Plymouth Marine Laboratory, the species buried themselves under gravel at the bottom of the tank. Under artificial light, that mirrored natural diffused daylight, adults showed increases in density (Denton & Gilpin-Brown, 1961). However, burying did not occur all the time during the periods of light exposure. If large cuttlefish were hungry, or if two males were in the same tank, they entered mid-water and remained there even under high light intensity. Sexual migration is not always determined by temperature. Individuals have been seen to leave warmer waters and arrive in April when waters are still only 10°C. It is thought that photoperiod plays a role in determining the time at which sexually mature individuals migrate back inshore (Boucaud-Camou & Boismery, 1991). The photoperiod is the length of daily illumination received by the individual (Koueta et al., 2005). In the English Channel, therefore, inshore migration usually occurs around the spring equinox when the daily photoperiod increases (Koueta and Boucaud-Camou, 2003). Hatching is also determined by the transition from light to dark (Koueta and Boucaud-Camou, 2003). Therefore, the absence of light is very important in synchronised hatching (Paulik et al., 1991) and reduced predation. Under a low photoperiod in a lab (8 h of light and 16 h of dark) growth rates were low for juveniles. Long photoperiods (24 h of light) in aquaculture resulted in a longer lifespan and larger individuals. However, gonad development appeared to be inhibited, allowing the animal to live longer but prohibited reproduction (Forsythe et al., 1994). Cuttlefish adjust their camouflage depending on light intensity. In extremely low light (<0.001 lux) cuttlefish do not camouflage but instead retract their dermal chromophores and choose a pale appearance, suggesting a low energy response (Buresch et al., 2015). Under high light intensity camouflage is increased with more energy consumption. A danger of the increase in an extended period of illumination is that more energy will have to be prioritised for camouflage and not other processes such as growth or reproduction (Buresch et al., 2015). Sensitivity assessment. Despite showing a behavioural response to light there is no evidence of death due to the effect of an introduction of light from an artificial source. Therefore, a resistance score of ‘High’ is recorded with ‘low’ confidence. Recoverability is probably ‘High’ and this species is therefore assessed as ‘Not Sensitive’ to the induction of light or shading. | HighHelp | HighHelp | Not sensitiveHelp |
Barrier to species movement [Show more]Barrier to species movementBenchmark. A permanent or temporary barrier to species movement over ≥50% of water body width or a 10% change in tidal excursion. Further detail EvidenceThere is evidence of gene flow between the English Channel and southern North Sea populations probably due to the mobility of this species. However, there is also clear genetic differences between the Atlantic and Mediterranean populations (Wolfram et al., 2006). Sensitivity assessment. Sepia officinalis migrates to deep water to breed and returns to coastal waters to spawn. A barrier to movement (e.g. barrage) is likely to prevent this migration and, since the species has a short lifespan, result in loss of the local population affected by the barrier. If the barrier is temporary then recovery may be rapid (a few years). However, a permanent barrier may exclude Sepia officinalis from the affected area. Therefore, in the worst-case scenario of a permanent barrier, resistance is probably None, and a resilience is ‘Very low’ (as the barrier is permanent) and sensitivity is assessed as ‘High’. However, confidence in the assessment is ‘low’. | NoneHelp | Very LowHelp | HighHelp |
Death or injury by collision [Show more]Death or injury by collisionBenchmark. Injury or mortality from collisions of biota with both static or moving structures due to 0.1% of tidal volume on an average tide, passing through an artificial structure. Further detail EvidenceCollision with the individual due to grounding by vessels is addressed under (abrasion). Therefore, this pressure is ‘Not relevant’. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Visual disturbance [Show more]Visual disturbanceBenchmark. The daily duration of transient visual cues exceeds 10% of the period of site occupancy by the feature. Further detail EvidenceSepia officinalis have the ability to perceive and differentiate different objects from their background as long as they differ in contrast from the background by at least 15% (Mäthger et al., 2006). They are thought to have just one visual pigment that has a maximum absorption of green light (492 nm) (Mäthger et al., 2006). Sepia officinalis are thought to have reasonable ‘night-vision’, due to their nocturnal hunting and feeding behaviour (Castro & Guerra, 1989). The species also appears to be colour-blind due to their inability to camouflage against and distinguish certain visual cues (Mäthger et al., 2006). When placed on a checkerboard of alternating blue and yellow shades of the same intensity, cuttlefish did not show a disruptive colouration suggesting it was unable to differentiate between the two colours of the same light intensity (Mäthger et al., 2006). It has been proposed (John Rundle, personal communication, cited in Tonkins et al., 2015) that dark figures leaning over tanks may be interpreted as predators by captive cuttlefish. This may lead to a defence response such as digging, swimming, and inking. Sensitivity assessment. The species has well-developed eyes so can detect movement sufficiently well to be susceptible to visual disturbance. However, the species will swim away or hide when any presence threatens, therefore, recovery is immediate. Visual cues may cause a behavioural response but due to the mobility of the species, the pressure is unlikely to cause mortality or interfere with reproduction. Hence, resistance is ‘High’, resilience is ‘High’, and the species is likely to be ‘Not sensitive’. | HighHelp | HighHelp | Not sensitiveHelp |
Biological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Genetic modification & translocation of indigenous species [Show more]Genetic modification & translocation of indigenous speciesBenchmark. Translocation of indigenous species or the introduction of genetically modified or genetically different populations of indigenous species that may result in changes in the genetic structure of local populations, hybridization, or change in community structure. Further detail EvidenceThere is evidence of gene flow between the English Channel and southern North Sea populations probably due to the mobility of this species. However, there is also clear genetic differences between the Atlantic and Mediterranean populations (Wolfram et al., 2006). Therefore, the translocation of specimens between Mediterranean and Atlantic population may affect the genotypes of the resident populations. However, there is no evidence that this has or is likely to occur and no evidence to suggest that it would be detrimental for the receiving population. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction or spread of invasive non-indigenous species [Show more]Introduction or spread of invasive non-indigenous speciesBenchmark. The introduction of one or more invasive non-indigenous species (INIS). Further detail EvidenceThe invasive blue crab Callinectes sapidus is originally from the coasts of the western Atlantic but has now reached European waters. It inhabits the same habitat niche of sandy bottoms at 35 m in depth as Sepia officinalis. In culture, it has also shown to actively feed on Sepia officinalis eggs. This crab has to potential to be a risk to Sepia officinalis but there is currently no evidence of actual introduction, therefore ‘No evidence’ is recorded (Catarina et al., 2017). | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction of microbial pathogens [Show more]Introduction of microbial pathogensBenchmark. The introduction of relevant microbial pathogens or metazoan disease vectors to an area where they are currently not present (e.g. Martelia refringens and Bonamia, Avian influenza virus, viral Haemorrhagic Septicaemia virus). Further detail EvidenceSome bacteria are not pathogenic but are in fact symbiotic and occur in the accessory rudimental gland in cuttlefish (Grigioni & Boucher-rodoni, 2002). It is thought that these bacteria are responsible for the colour change in the gland from white to bright red/orange which signifies the individual is sexually mature (Grigoni & Boucher-Rodoni, 2002). Sepia officinalis also has naturally occurring Gammaproteobacteria, e.g. Vibrionaceae, in the gills and oesophagus. These bacteria appear to be resilient to the common animal antibiotic enrofloxacin (Lutz et al., 2018). A common infection spot in Sepia officinalis is in the digestive tract and stomach epithelium (Hanlon & Forsythe, 1990). A virus similar in structure to a retrovirus in vertebrates was found in the stomachs of wild cuttlefish (Hanlon & Forsythe, 1990). Microbes were recorded on the outside of Sepia officinalis egg cases that reduced the oxygen available to diffuse into the egg (Cronin & Seymour, 2000). The coccidian Aggregata eberthi appears to use the digestive tract of the cuttlefish as part of their sexual reproduction (Gestal et al., 2002). This has been known to lead to an increase in gut infections, which reduces the function of the digestive system and limits the absorption of nutrients (Gestal et al., 2002). However, there is little evidence of direct mortality as a result of microbial infection (Hochberg, 1990). Fungal infections can appear on Sepia officinalis but these are usually the result of trauma to the skin or a weakening of the immune system (Harms et al., 2006). Cells known as ovoid cells in the heart tissue are known to target bacterial toxins in the blood. This shows a strong immune response to microbial infection and suggests a tolerance to them (Beuerlin & Schipp, 1998). Despite their resistance, inflammation and secondary bacterial infections occur generally in cuttlefish that have sustained trauma or mantle lesions. Septicaemia (Vibrio spp.) caused by mantle erosion from trauma and/or collision resulted in the death of 4/7 cultured cuttlefish under standard culturing conditions (Sherrill et al., 2000). Sensitivity assessment. Sepia officinalis appear to be relatively tolerant to microbial infections but once damaged due to trauma appear less resistant, resulting in a score of ‘Medium’. Recovery is probably ‘Medium’ and sensitivity is, therefore ‘Medium’. | MediumHelp | MediumHelp | MediumHelp |
Removal of target species [Show more]Removal of target speciesBenchmark. Removal of species targeted by fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidenceIn many areas, Sepia officinalis is fished at the maximum sustainable rate but in others, there has been a recent decline in captures in heavily fished areas. It is the most abundant cephalopod species fished in the North East Atlantic with annual landings ranging from 40,000-50,000 (ICES, 2009). The UK cuttlefish landings for UK vessels in 2015 was ca. 6000 tonnes (MMO, 2016; cited in ICES, 2017(b)). The primary mode of fishing is trawling but cuttlefish are also caught by artisanal fisheries using pots and traps (ICES, 2003). The peak inshore fishing season, unfortunately, coincides with the peak spawning period meaning adults have a limited time to reproduce and replenish the subsequent population before they are fished (ICES, 2003). In inshore French waters, Sepia officinalis is harvested by traps that selectively remove males prior to reproduction, trawls that capture a high percentage of females and juveniles, and nets that capture an even ratio of adult males and females (du Sel et al., 1997). Females have been shown to mate with multiple males meaning a reduction in males may not affect the fecundity of the population drastically. Sepia officinalis eggs are removed unintentionally as the females attach them to cuttletraps from March to September. The fishermen, fishing for adult cuttlefish, do not tend to place the traps straight back into the ocean and instead leave the traps and eggs on the pontoon to be desiccated (Blanc & Daguzan, 1998). Due to the lack of generation overlap, the mortality of eggs is detrimental to the recruitment of the population. There is an issue of high discarding rates within the cephalopod fisheries. In 2008, an estimated 306 tonnes of small cuttlefish were discarded in the south-west of England (CEFAS; cited in Revill, 2011). From interviews with fishermen, a major reason for discarding juveniles is the hope that they will grow bigger and to a more valuable size for the following years capture (Revill, 2011). Sepia officinalis from a large beam trawler operating in the offshore waters of south-west UK were examined during a single fishing trip for signs of damage and survival (Revill, 2011). Analysis showed that 16% of these small cuttlefish (<15 cm) died later after their injuries from capture, 68% died whilst in the trawl or on the deck, and only 16% survived capture and deck sorting and were expected to survive discarding (Revill, 2011). There is currently no routine monitoring or assessment scheme in place for the English Channel Sepia officinalis stock (Gras et al., 2014). During the sea trials, large cuttlefish were able to survive the trauma of trawling and deck sorting more than the delicate smaller cuttlefish. The damage to the smaller cuttlefish was damage to the skin and even internal cuttlebone suggesting smaller cuttlefish are more fragile and weaker than adults (Revill, 2011). As there is no current minimum landing size of cuttlefish in the UK. Therefore small cuttlefish are more vulnerable to catch and discarding than adults (British Sea Fishing, 2018). Landings are also increasing globally. For example, in the English Channel, total annual landings have increased from 430t in 1992 to 17,400t in 2014 (Gras et al., 2016). Dunn (1999) suggested that as the market value is increased, cephalopods could becoming more popular for fishermen, which may begin to hinder Sepia officinalis stocks in the future. Sensitivity assessment. Sepia officinalis have ‘Low’ resistance to removal. Resilience is likely to be ‘Medium’ as there is no current recorded global decline in stocks. However, stocks of Sepia officinalis are currently able to just about continue replenishing the current population but if fishing pressures increase the likelihood of replenishment will be reduced (ICES, 2017(b)). Sensitivity is, therefore, 'Medium’. | LowHelp | MediumHelp | MediumHelp |
Removal of non-target species [Show more]Removal of non-target speciesBenchmark. Removal of features or incidental non-targeted catch (by-catch) through targeted fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidenceExtraction of another species will not have a strong effect on this species as their prey is very varied and they have no dependence on one particular species. Sepia officinalis have a varied diet from amphipods to gobies and sand eels. In extreme cases, they may even become cannibalistic, preying on smaller individuals (Castro & Guerra, 1990). Prey size is also selective, with adult Sepia officinalis choosing fish 25-80% and crabs 20-40% the size of their mantle length (Blanc et al., 1999). Predators of Sepia officinalis include; sharks, lobsters and other cephalopods (Catarina et al., 2007). The extraction of non-target species is not thought to affect Sepia officinalis from a biological point of view. Instead, the biggest risk to the species due to this removal is; removal of eggs by accident (see abrasion) and by-catch. In the Mersin province, Turkey, 10g of Sepia officinalis were caught as by-catch using one trawl from September 2004 to April 2005. The target fish were; mullet, goatfish and shrimp (Atar & Malal, 2010). In Algarve (southern Portugal), Sepia officinalis are occasionally caught in tuna traps (Neves dos Santos et al., 2002). Many eggs are laid on cuttletraps during spawning periods. When removed from water fishermen are not interested in collecting the eggs. Instead, they are usually left on the pontoon until further use resulting in either mortality or significant damage (Blanc & Daguzan, 1998). Sensitivity assessment. Resistance is probably ‘Medium’ but with ‘Low’ confidence due to a lack of available by-catch figures. Resilience is probably ‘Medium’ and sensitivity is ‘Medium’ | MediumHelp | MediumHelp | MediumHelp |
Importance review
Policy/legislation
| Designation | Support |
|---|---|
| IUCN Red List | Least Concern (LC) |
Status
| National (GB) importance | - | Global red list (IUCN) category | Least Concern (LC) |
Non-native
| Parameter | Data |
|---|---|
| Native | Native |
| Origin | - |
| Date Arrived | N/A |
Importance information
Cephalopods are known for their intelligence (Hastie et al., 2009). Sepia officinalis has the ability to camouflage and undertake learned behaviour. Sepia officinalis can also alter their buoyancy by controlling how much liquid is placed into or out of the cuttlebone (Denton & Gilpin-Brown, 1961). Dunn (1999) and Gutowska et al., (2008) reported that an increase in pCO2, and therefore a decrease in pH, appeared to have no effect on the ability to calcify the cuttlebone. Therefore, Sepia officinalis may be well adapted to deal with the issue of ocean acidification to a moderate level. In terms of ecological value, Sepia officinalis appears to play a dominant role in the marine food chain (Boletzky & Hanlon, 1983). Sepia officinalis has also been shown to have antimicrobial agents in their body tissue and cuttlebone. These agents were able to increase the survival rates of CLP-septic (cecal ligation and puncture ) rates by 66.7 – 83.33%. This may be due to the chelating activity of the body tissues which may prevent the further growth of pathogens (Soilman et al., 2016).
Ethics. Cephalopods are “recognized as advanced organisms capable of pain and suffering and were recently added to the list of protected animal groups covered by European welfare legislation” (Directive 2010/63/EU). Since 1st January 2013, all cephalopods have been protected under UK/EU law (A (SP) A1986, European Directive 2010/63/EU).
Fishing. Sepia officinalis is the most abundant cephalopod resource in the North East Atlantic. Cephalopod annual landings have ranged from 40,000 – 50,000 tonnes from 1999-2009 (ICES, 2009). Of these landings, cuttlefish accounted for 16,000 to 24,000 tonnes annually (Hastie et al., 2009). Sepia officinalis accounted for a large proportion of these landings. It is also one of the three most valuable resources for the English Channel Fisheries (Gras et al., 2014). The UK cuttlefish landings for UK vessels in 2015 were ca. 6000 tonnes (MMO, 2016; cited in ICES, 2017(b)). In the English Channel, landings of Sepia officinalis have increased from 430 t in 1992 to 17,400 t in 2014 (Gras et al., 2016). This increase was driven by an increase in the market value of cuttlefish during the 1980s from £0.40 per kg to £1.10 per kg (Dunn, 1999). In the Archipelago of Madeira, the total landings appear to fluctuate annually. For example, the total landings of Sepia officinalis in Madeira ranged from 1978 t in 2013 to 1052 t in 2015 (Guerra et al., 2016b). Due to the commercial value and lack of landing restrictions, individuals are only discarded if damaged. In a southern otter trawl, 77% of the landed Sepia officinalis were discarded, suggesting high levels of damage occurring from these trawls (Guerra et al., 2016b). However, the species is also recorded as one of the species with high levels of unreported catches (Batista et al., 2015).
Protection. The English Channel is dominated by offshore trawlers accounting for 90% of the UK landings of cephalopods, whereas inshore traps contribute to only 5% of UK landings (ICES, 2003). Inshore landings are higher between March and June, corresponding to the peak in the spawning season. However, this means that Sepia officinalis are fished during their most vulnerable spawning period, vital for recruitment (ICES, 2003). There is currently no routine monitoring or assessment scheme in place for the English Channel Sepia officinalis stock (Gras et al., 2014, ICES, 2017(b)). Cephalopod fisheries are continuing to expand in Europe, declining marine stocks (Turan & Yaglioglu, 2010). Cephalopods, including Sepia officinalis, have been reported to benefit from local bans on trawling, increasing mesh size and banning fishing in certain shore areas from operating within three nautical miles of the shore (Gras et al., 2014). Protection has also occurred through the trial of more selective fishing gears including net modifications (e.g. larger mesh size). The results showed the net modification could reduce the amount of juvenile fish, cuttlefish included, caught by 57% (CEFAS, 2009). Marine protected areas do not appear to show effective long-term protection for this species due to its mobile and migratory nature (ICES, 2015(b)). It may be effective, however, solely as a protection for the spawning zone, ensuring successful recruitment (ICES, 2015(b)).
No specific management measure has been introduced in the UK to date to maintain and manage commercially important English Channel cuttlefish stock. There is no minimum size limit for shore caught cephalopods and it is a non-quota species in the UK (British Sea Fishing, 2018). There is, however, a minimum conservation reference size in UK waters for the octopus Octopus vulgaris of 750g (Marine Management Organisation, 2017) meaning at least one species of cephalopod is currently managed in the UK. In Spain, there is a minimum landing size of 8 cm for small-scale fisheries and during the months of May to July and fishing must occur above 5 m depth for boats less than 2.5 GRT (RAGG, 2015; cited in Guerra et al., 2016(b)). There is a similar size/weight restriction enforced in Portugal for Sepia officinalis (Hastie et al., 2009). Sepia officinalis is currently described as ‘least concern’ on the IUCN red list (IUCN, 2009).
Aquaculture. Sepia officinalis has been successfully cultured in both aquaria and aquaculture. The species is used for biomedical and environmental research and is thought to be the most easily cultured cephalopod species (Forsythe et al., 1994, Domingues et al., 2001, Koueta et al., 2006).
Bibliography
André, M., Solé, M., Lenoir, M., Durfort, M., Quero, C., Mas, A., Lombarte, A., van der Schaar, M., López-Bejar, M., Morell, M., Zaugg, S. & Houégnigan, L., 2011. Low-frequency sounds induce acoustic trauma in cephalopods. Frontiers in Ecology and the Environment, 9, 489-493.
Atar, H.H. & Malal, S., 2010. Determination of bycatch and discard catch rates on trawl fishing in Mersin-Anamur fishing ground. Journal of Food Agriculture and Environment, 8, 348-352.
Barratt, I. & Allcock, L., 2012. Sepia officinalis. The IUCN Red List of Threatened Species 2012: e.T162664A939991. Accessible from: http://dx.doi.org/10.2305/IUCN.UK.2012-1.RLTS.T162664A939991.en
Bedore, C.N., Kajiura, S.M. & Johnsen, S., 2015. Freezing behaviour facilitates bioelectric crypsis in cuttlefish faced with predation risk. Proceedings of the Royal Society B: Biological Sciences, 282: 20151886
Bettencourt, V. & Guerra, A., 1999. Carbon- and oxygen-isotope composition of the cuttlebone of Sepia officinalis: a tool for predicting ecological information? Marine Biology, 133, 651-657.
Beuerlein, K. & Schipp, R., 1998. Cytomorphological aspects on the response of the branchial heart complex of Sepia officinalis L. (Cephalopoda) to xenobiotics and bacterial infection. Tissue and Cell, 30(6), 662-671.
Blanc, A. & Daguzan, J., 1998. Artificial surfaces for cuttlefish eggs (Sepia officinalis L.) in Morbihan Bay, France. Fisheries Research, 38, 225-231.
Blanc, A. & Daguzan, J., 1999. Young cuttlefish Sepia officinalis (Mollusca: Sepiidae) in the Morbihan Bay (south Brittany, France): accessory prey of predators. Journal of the Marine Biological Association of the U.K., 79, 1133-1134.
Blanc, A., du Sel, G.P. & Daguzan, J., 1999. Relationships between length of prey/predator for the most important prey of the cuttlefish Sepia officinalis L. (Mollusca: Cephalopoda). Malacologia, 41(1), 139-145.
Blanc, A., du Sel, P. & Daguzan, J., 1998. Habitat and diet of early stages of Sepia officinalis L. (Cephalopoda) in Morbihan Bay, France. Journal of Molluscan Studies, 64, 263-274.
Bloor, I. & Jackson, E. 2014. Seafish Ecological risk assessment of South West fisheries: Cephalopod ecosystem component. SR672 Ecological Risk Assessment of the effects of fishing for South West fisheries; ICES Divisions VII e,f,g & h; Supporting information, 12-15. Avaliable: http://www.seafish.org/media/publications/SR672ERAEFSupporting_information.pdf
Bloor, I., 2016. The current and changing role of physico-chemical factors and cues in the embryonic and early life stage development of the common cuttlefish (Sepia Officinalis). Vie et milieu, 66(1), 81-95.
Bloor, I.S.M., Attrill, M.J. & Jackson, E.L., 2013. A review of the factors influencing spawning, early life stage survival and recruitment variability in the common cuttlefish (Sepia officinalis). Advances in Marine Biology, 65, 1-65.
Bloor, I.S.M., Wearmouth, V.J., Cotterell, S.P., McHugh, M.J., Humphries, N.E., Jackson, E.L., Attrill, M.J. & Sims, D.W., 2013b. Movements and behaviour of European common cuttlefish Sepia officinalis in English Channel inshore waters: first results from acoustic telemetry. Journal of Experimental Marine Biology and Ecology, 448, 19-27.
Boal, J.G. & Golden, D.K., 1999. Distance chemoreception in the common cuttlefish, Sepia officinalis (Mollusca, Cephalopoda). Journal of Experimental Marine Biology and Ecology, 235, 307-317.
Boal. J.G., 1997. Female choice of males in cuttlefish (Mollusca: Cephalopoda). Behaviour, 134, 975-988.
Boletzky, S.v., 1987. Fecundity variation in relation to intermittent or chronic spawning in the cuttlefish, Sepia officinalis L. (Mollusca, Cephalopoda). Bulletin of Marine Science, 40, 382-388
Bouchaud, O., 1991. Energy consumption of the cuttlefish Sepia officinalis L. (Mollusca: Cephalopoda) during embryonic development, preliminary results. Bulletin of Marine Science, 49, 333-340.
Boyle, P.R. (ed.), 1983. Cephalopod Life Cycles, vol 1. Species Accounts. London: Academic Press Inc. (London) Ltd.
Boyle, P.R., 1990. Cephalopod biology in the fisheries context. Fisheries Research. 8, 303- 321.
British Sea Fishing, 2018. Common Cuttlefish. Other sea creature species. Cited: (24/10/18). Available from: http://britishseafishing.co.uk/common-cuttlefish/
Buresch, K.C., Ulmer, K.M., Akkaynak, D., Allen, J.J., Mäthger, L.M., Nakamura, M. & Hanlon, R.T., 2015. Cuttlefish adjust body pattern intensity with respect to substrate intensity to aid camouflage, but do not camouflage in extremely low light. Journal of Experimental Marine Biology and Ecology, 462, 121-126.
Campbell, A., 1994. Seashores and shallow seas of Britain and Europe. London: Hamlyn.
Capaz, J.C., Tunnah, L., MacCormack, T.J., Lamarre, S.G., Sykes, A.V. & Driedzic, W.R., 2017. Hypoxic induced decrease in oxygen consumption in cuttlefish (Sepia officinalis) is associated with minor increases in mantle octopine but no changes in markers of protein turnover. Frontiers in Physiology, 8, 344.
Castro, B.G. & Guerra, A., 1990. The diet of Sepia officinalis (Linnaeus, 1758) and Sepia elegans (D'Orbigny, 1835) (Cephalopoda, Sepioidea) from the Ria de Vigo (NW Spain). Scientia Marina, 54(4), 375-388.
CEFAS, 2009. Project 50%. Defra. Final report, available from: http://www.seafish.org
Chaddha, R. 2007. Antomy of a cuttlefish. NOVA (online). Accessed: http://www.pbs.org/wgbh/nova/nature/anatomy-cuttlefish.html (17/10/18).
Challier, L., Dunn, M.R., & Robin, J.-P., 2005. Trends in age-at-recruitment and juvenile growth of cuttlefish, Sepia officinalis, from the English Channel. ICES Journal of Marine Science, 62, 1671-1682.
Challier, L., Royer, J. & Robin, J.P., 2002. Variability in age-at-recruitment and early growth in English Channel Sepia officinalis described with statolith analysis. Aquatic Living Resources, 15(5), 303-311.
Compton, A. & Wiley. L, 2011. "Sepia officinalis" (On-line), Animal Diversity Web. Accessed: https://animaldiversity.org/accounts/Sepia_officinalis/ (17/10/18)
Cooke, G. & Tonkins, B., 2015. Behavioural indicators of welfare exhibited by the common European cuttlefish (Sepia officinalis). Journal of Zoo and Aquarium Research, 3, 157-162.
Cronin, E.R. & Seymour, R.S., 2000. Respiration of the eggs of the giant cuttlefish Sepia apama. Marine Biology, 136, 863-870.
Danis, B., Bustamante, P., Cotret, O., Teyssié, J.L., Fowler, S.W. & Warnau, M., 2005. Bioaccumulation of PCBs in the cuttlefish Sepia officinalis from the seawater, sediment and food pathways. Environmental Pollution, 134, 113-122.
Denis, V. & Robin, J.-P., 2001. Present status of the French Atlantic fishery for cuttlefish (Sepia officinalis). Fisheries Research, 52, 11-22.
Denton, E.J. & Gilpin-Brown, J.B., 1961. The effect of light on the buoyancy of the cuttlefish. Journal of the Marine Biological Association of the United Kingdom, 41, 343-350.
Diaz, R.J. & Rosenberg, R., 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology: an Annual Review, 33, 245-303.
Domingues, P.M., Kingston, T., Sykes, A. & Andrade, J.P., 2001. Growth of young cuttlefish, Sepia officinalis (Linnaeus 1758) at the upper end of the biological distribution temperature range. Aquaculture Research, 32, 923-930.
du Sel, G.P. & Daguzan, J., 1997. A note on sex ratio, length and diet of a population of cuttlefish Sepia officinalis L. (Mollusca: Cephalopoda) sampled by three fishing methods. Fisheries Research, 32(2), 191-195.
Dunn, M.R., 1999. Aspects of the stock dynamics and exploitation of cuttlefish, Sepia officinalis (Linnaeus, 1758), in the English Channel. Fisheries Research, 40, 277-293.
Eberhard. W. G., 1996. Female control sexual selection by cryptic female choice. Monographs in Behavior and Ecology. pp. 472
Enever, R., Revill, A. & Grant, A., 2007. Discarding in the English Channel, Western approaches, Celtic and Irish seas (ICES subarea VII). Fisheries Research, 86, 143-152.
Ferguson, G., Messenger, J. & Budelmann, B., 1994. Gravity and light influence the countershading reflexes of the cuttlefish Sepia officinalis. Journal of Experimental Biology, 191, 247-256.
Forsythe, J.W., DeRusha, R.H. & Hanlon, R.T., 1994. Growth, reproduction and life span of Sepia officinalis (Cephalopoda: Mollusca) cultured through seven consecutive generations. Journal of the Zoological Society of London, 233, 175-192.
Frank, M.G., Waldrop, R.H., Dumoulin, M., Aton, S. & Boal, J.G., 2012. A preliminary analysis of sleep-like states in the cuttlefish Sepia officinalis. PLoS ONE, 7, e38125.
Gauvrit, E., Goff, R.L. & Daguzan, J., 1997. Reproductive cycle of the cuttlefish, Sepia officinalis (l.) in the northern part of the bay of biscay. Journal of Molluscan Studies, 63, 19-28.
Gestal, C., Guerra, A., Pascual, S. & Azevedo, C., 2002. On the life cycle of Aggregata eberthi and observations on Aggregata octopiana (Apicomplexa, Aggregatidae) from Galicia (NE Atlantic). European Journal of Protistology, 37, 427-435.
Goff, R.L. & Daguzan, J., 1991. Growth and life cycles of the cuttlefish Sepia officinalis L. (Mollusca: Cephalopoda) in south brittany (France). Bulletin of Marine Science, 49, 341-348.
Gras, M., Roel, B.A., Coppin, F., Foucher, E. & Robin, J.-P., 2014. A two-stage biomass model to assess the English Channel cuttlefish (Sepia officinalis L.) stock. ICES Journal of Marine Science, 71, 2457-2468.
Gras, M., Safi, G., Lebredonchel, H., Quinquis, J., Foucher, É., Koueta, N. & Robin, J.-P., 2016. Stock structure of the English Channel common cuttlefish Sepia officinalis (Linnaeus, 1758) during the reproduction period. Journal of the Marine Biological Association of the United Kingdom, 96, 167-176.
Grigioni, S. and Boucher-Rodoni, R., 2002. Symbiotic associations in Sepia officinalis. Bulletin of Marine Science, 71,1124.
Guerra, Á., Hernández-Urcera, J., Garci, M.E., Sestelo, M., Regueira, M., Gilcoto, M. & González, Á.F., 2016. Spawning habitat selection by the common cuttlefish Sepia officinalis in the Cíes Islands (Northwest Spain). Fisheries Research, 183, 44-54.
Guerra, A. & Castro, B., 1988. On the life cycle of Sepia officinalis (Cephalopoda, Sepioidea) in the ria de Vigo (NW Spain). Muelle de Bouzas, 29, 395-405.
Guerra, A., 2006. Ecology of Sepia officinalis. Vie et milieu, 56, 97-107.
Gutowska, M.A. & Melzner, F., 2009. Abiotic conditions in cephalopod (Sepia officinalis) eggs: embryonic development at low pH and high pCO2. Marine Biology, 156, 515-519.
Gutowska, M.A., Portner, H.O. & Melzner, F., 2008. Growth and calcification in the cephalopod Sepia officinalis under elevated seawater CO2. Marine Ecology Progress Series, 373, 303-309.
Häfker, N.S. 2012. Effects of hypoxia and hypercapnia on blood and tissue physiology of the common cuttlefish Sepia officinalis. Masters thesis. Universtität Bremen.
Hanlon, R. & Forsythe, J.W., 1990. Diseases caused by microorganisms. Diseases of Marine Animals: Cephalopoda to Urochordata Kinne, 3, 23-46
Hanlon, R. T. & Messenger, J. B., 1996. Cephalopod Behaviour. Cambridge: Cambridge University Press.
Hanlon. R.Y., Ament. S.A., Gabr. H., 1999. Behavioral aspects of sperm competition in cuttlefish, Sepia officinalis (Sepioidea: Cephalopoda). Marine Biology, 134, 719-728.
Harms, C.A., Lewbart, G.A., McAlarney, R., Christian, L.S., Geissler, K. & Lemons, C., 2006. Surgical Excision of Mycotic (Cladosporium sp.) Granulomas from the Mantle of a Cuttlefish (Sepia officinalis). Journal of Zoo and Wildlife Medicine, 37, 524-530.
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Ho, J.-S., 1983. Metaxymolgus longicauda (Claus), a copepod associated with the cuttle-fish, Sepia officinalis L. Journal of the Marine Biological Association of the United Kingdom, 63(1), 199-203.
Hochberg. F. G.,1990. Diseases caused by protistans and metazoans. In Kinne: Diseases of Marine Animals. Vol III. Diseases of Mollusca: Cephalopoda, Hamburg: pp. 47-202.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
ICES, 1994. Report of the study group on the life history assessment of Cephalopods. Copenhagen-Denmark ICES, K:7, 32.
ICES, 2017(b). Interim report of the working group on cephalopod fisheries and life history (WGCEPH). ICES CM 2017/SSGEPD:12. 132 pp. Available from: http://ices.dk/sites/pub/Publication Reports/Expert Group Report/SSGEPD/2017/01 WGCEPH - Report of the Report of the Working Group on Cephalopod Fisheries and Life History.pdf
ICES. 2012. Report of the Working Group on Cephalopod Fisheries and Life History (WGCEPH), 27–30 March 2012, Cadiz, Spain. ICES CM 2012/SSGEF:04, pp. 241 Available from: http://www.ices.dk/sites/pub/Publication Reports/Expert Group Report/SSGEF/2012/WGCEPH12.pdf
Johansen, K., Brix, O., Kornerup, S. & Lykkeboe, G., 1982. Factors affecting oxygen uptake in the cuttlefish, Sepia officinalis. Journal of the Marine Biological Association of the United Kingdom, 62, 187-191.
Jones, N.J.E., Ridgway, I.D. & Richardson, C.A., 2009. Transport of cuttlefish, Sepia officinalis, eggs under dry and damp conditions. Journal of Molluscan Studies, 75, 192-194.
Jozet-Alves, C., Bertin, M. & Clayton, N.S., 2013. Evidence of episodic-like memory in cuttlefish. Current Biology, 23, R1033-R1035.
Keller, S., Valls, M., Hidalgo, M. & Quetglas, A., 2014. Influence of environmental parameters on the life-history and population dynamics of cuttlefish Sepia officinalis in the western Mediterranean. Estuarine, Coastal and Shelf Science, 145, 31-40.
Komak, S., Boal, J.G., Dickel, L. & Budelmann, B.U., 2005. Behavioural responses of juvenile cuttlefish (Sepia officinalis) to local water movements. Marine and Freshwater Behaviour and Physiology, 38, 117-125.
Koueta, N. & Boucaud-Camou, E., 2001. Basic growth relations in experimental rearing of early juvenile cuttlefish Sepia officinalis L. (Mollusca: Cephalopoda). Journal of Experimental Marine Biology and Ecology, 265, 75-87.
Koueta, N., Castro, B.G. & Boucaud-Camou, E., 2000. Biochemical indices for instantaneous growth estimation in young cephalopod Sepia officinalis L. ICES Journal of Marine Science, 57, 1-7.
Kunc, H.P., Lyons, G.N., Sigwart, J.D., McLaughlin, K.E. & Houghton, J.D.R., 2014. Anthropogenic noise affects behavior across sensory modalities. The American Naturalist, 184, 93-100.
Lutz, H.L., Ramirez-Puebla, T., Abbo, L., Schlundt, C., Sjaarda, A.K., Durand, A., Hanlon, R.T., Gilbert, J. & Mark Welch, J.L., 2018. A simple microbiome in esophagus and gills of the European common cuttlefish, Sepia officinalis. bioRxiv 440677; DOI: https://doi.org/10.1101/440677
Mäthger, L.M., Barbosa, A., Miner, S. & Hanlon, R.T., 2006. Color blindness and contrast perception in cuttlefish (Sepia officinalis) determined by a visual sensorimotor assay. Vision Research, 46, 1746-1753.
Mangold, K., 1966. Sepia officinalis de la Mer Catalane. Vie et Milieu, 17, 961-1012.
Marine Management Organisation (MMO), 2017. Minimum conservation reference sizes (MCRS) in UK waters. Gov.UK. Available from: https://www.gov.uk/government/publications/minimum-conservation-reference-sizes-mcrs/minimum-conservation-reference-sizes-mcrs-in-uk-waters
Mattacola, A.D., Maddock, L. & Denton, E.J., 1984. Weights and lengths of Sepia officinalis trawled by the laboratory's boats 1978-1983. Journal of the Marine Biological Association, 64(3), 735-737.
Melzner, F., Bock, C., Pörtner, H.O. 2006. Temperature-dependent oxygen extraction from the ventilatory current and the costs of ventilation in the cephalopod Sepia officinalis. Journal of Comparative Physiology B-Biochemical Systemic and Environmental Physiology, 176, 607-621
Miramand, P. & Bentley, D., 1992. Concentration and distribution of heavy metals in tissues of two cephalopods, Eledone cirrhosa and Sepia officinalis, from the French coast of the English Channel. Marine Biology, 114, 407-414.
Naud, M.J., Shaw, P.W., Hanlon, R.T. & Havenhand, J.N., 2005. Evidence for biased use of sperm sources in wild female giant cuttlefish (Sepia apama). Proceedings of the Royal Society B: Biological Sciences, 272, 1047-1051
Neves dos Santos, M., Jorge Saldanha, H. & Garcia, A., 2002. Observations on by-catch from a tuna trap fishery off the Algarve (southern Portugal). ICCAT, 54, 1726-1732.
Nixon, M., 1985. Capture of prey, diet and feeding of Sepia officinalis and Octopus vulgaris (Mollusca: Cephalopoda) from hatching to adult. Vie et Milieu, 35(3/4), 255-261.
O'Brien, C.E., Jozet-Alves, C., Mezrai, N., Bellanger, C., Darmaillacq, A.-S. & Dickel, L., 2017. Maternal and Embryonic Stress Influence Offspring Behavior in the Cuttlefish Sepia officinalis. Frontiers in Physiology 8, 981.
Oellermann, M., Pörtner, H.O. & Mark, F.C., 2012. Mitochondrial dynamics underlying thermal plasticity of cuttlefish (Sepia officinalis) hearts. The Journal of Experimental Biology, 215, 2992.
Oliveira, C., Grano-Maldonado, M., Gonçalves, R., Frias, P. & Sykes, A., 2017. Preliminary results on the daily and seasonal rhythms of cuttlefish Sepia officinalis (linnaeus, 1758) locomotor activity in captivity. Fishes, 2, 9.
Pérez-Losada, M., Guerra, A. & Sanjuan, A., 1999. Allozyme differentiation in the cuttlefish Sepia officinalis (Mollusca: Cephalopoda) from the NE Atlantic and Mediterranean. Heredity, 83, 280-289.
Pérez-Losada, M., Guerra, A., Carvalho, G.R., Sanjuan, A. & Shaw, P.W., 2002. Extensive population subdivision of the cuttlefish Sepia officinalis (Mollusca: Cephalopoda) around the Iberian Peninsula indicated by microsatellite DNA variation. Heredity, 89, 417.
Palmegiano, G.B. & D'Apote, M.P., 1983. Combined effects of temperature and salinity on cuttlefish (Sepia officinalis L.) hatching. Aquaculture, 35, 259-264.
Pascual. E., 1978. Crecimiento y alimentación de tres generaciones de Sepia officinalis en cultivo. Invest Pesqu, 42, 421-441.
Paulij, W.P., Bogaards, R.H. & Denuce, J.M., 1990. Influence of salinity on embryonic development and the distribution of Sepia officinalis in the Delta Area (South Western part of The Netherlands). Marine Biology, 107, 17-23.
Paulij, W.P., Herman, P.M.J., Roozen, M.E.F. & Denuce, J.M., 1991. The influence of photoperiodicity on hatching of Sepia officinalis. Journal of the Marine Biological Association of the United Kingdom, 71, 665-678.
Pierce, G.J., Boyle, P.R., Hastie, L.C. & Shanks, A.M., 1994. Distribution and abundance of the fished population of Loligo forbesii in UK waters: analysis of fishery data. Special Issue: Fishery Biology of Northeast Atlantic Squid, Fisheries Issue, 21, 193-216.
Poirier, R., Chichery, R. & Dickel, L., 2004. Effects of rearing conditions on sand digging efficiency in juvenile cuttlefish. Behavioural Processes, 67, 273-279.
Robin, J. P., Roberts, M., Zeidberg, L., Bloor, I., Rodriguez, A., Briceño, F., Downey, N., Mascaró, M., Navarro, M., Guerra, A., Hofmeister, J., Barcellos, D.D., Lourenço, S.A.P., Roper, C.F.E., Moltschaniwskyj, N.A., Green, C.P. & Mather, J., 2014. Transitions During Cephalopod Life History: The Role of Habitat, Environment, Functional Morphology and Behaviour. In Vidal, E.A.G. (ed.) Advances in Marine Biology, 64, 361-437.
Rocha, F., Guerra, Á., & González, A. F. 2001. A review of reproductive strategies in cephalopods. Biological Reviews, 76, 291–304.
Rodhouse, P.G.K., Pierce, G.J., Nichols, O.C., Sauer, W.H.H., Arkhipkin, A.I., Laptikhovsky, V.V., Lipiński, M.R., Ramos, J.E., Gras, M., Kidokoro, H., Sadayasu, K., Pereira, J., Lefkaditou, E., Pita, C., Gasalla, M., Haimovici, M., Sakai, M. & Downey, N., 2014. Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries. In Vidal, E.A.G. (ed.) Advances in Marine Biology, 64, 99-233.
Rodhouse, P. G., Nigmatullin, C. M., 1996. Role as consumers. Philosophical Transactions of the Royal Society B: Biological Sciences, 351, 1003- 1022.
Romagny, S., Darmaillacq, A.-S., Guibé, M., Bellanger, C. & Dickel, L., 2012. Feel, smell and see in an egg: emergence of perception and learning in an immature invertebrate, the cuttlefish embryo. The Journal of Experimental Biology, 215, 4125-4130.
Roper, C.F.E., Sweeney, M.J. & Nauen, C.E., 1984. FAO species catalogue. Vol. 3. Cephalopods of the world. An annotated and illustrated catalogue of species of interest to fisheries. FAO Fisheries Synopsis, 125, 3, 277. Avaliable: http://www.fao.org/docrep/009/ac479e/ac479e00.htm
Rosa, I.C., Raimundo, J., Lopes, V.M., Brandão, C., Couto, A., Santos, C., Cabecinhas, A.S., Cereja, R., Calado, R., Caetano, M. & Rosa, R., 2015. Cuttlefish capsule: an effective shield against contaminants in the wild. Chemosphere, 135, 7-13.
Royer, J., Pierce, G.J., Foucher, E. & Robin, J.P., 2006. The English Channel stock of Sepia officinalis: modelling variability in abundance and impact of the fishery. Fisheries Research, 78, 96-106.
Samson, J.E., Mooney, T.A., Gussekloo, S.W.S. & Hanlon, R.T., 2014. Graded behavioral responses and habituation to sound in the common cuttlefish Sepia officinalis. The Journal of Experimental Biology, 217, 4347.
Sherrill, J., Spelman, L.H., Reidel, C.L. & Montali, R.J., 2000. Common cuttlefish (Sepia officinalis) mortality at the national zoological park: Implications for clinical management. Journal of Zoo and Wildlife Medicine, 31, 523-531.
Shohet, A.J., Baddeley, R.J., Anderson, J.C., Kelman, E.J. & Osorio, D., 2006. Cuttlefish responses to visual orientation of substrates, water flow and a model of motion camouflage. Journal of Experimental Biology, 209, 4717.
Sobrino, I., Silva, L., Bellido, J. & Ramos, F., 2002. Rainfall, river discharges and sea temperature as factors affecting abundance of two coastal benthic cephalopod species in the Gulf of Cádiz (SW Spain). Bulletin of Marine Science -Miami, 71, 851-856.
Solé, M., Lenoir, M., Durfort, M., López-Bejar, M., Lombarte, A., van der Schaar, M. & André, M., 2013. Does exposure to noise from human activities compromise sensory information from cephalopod statocysts? Deep Sea Research Part II: Topical Studies in Oceanography, 95, 160-181.
Solé, M., Sigray, P., Lenoir, M., van der Schaar, M., Lalander, E. & André, M., 2017. Offshore exposure experiments on cuttlefish indicate received sound pressure and particle motion levels associated with acoustic trauma. Scientific Reports, 7, 45899
Soliman, A.M., Fahmy, S.R., Sayed, A.A. & Abd El-Latif, A.A., 2016. New insights into sepsis therapy using Sepia Officinalis. Jundishapur journal of microbiology, 9
Tonkins, B.M., Tyers, A.M. & Cooke, G.M., 2015. Cuttlefish in captivity: an investigation into housing and husbandry for improving welfare. Applied Animal Behaviour Science, 168, 77-83.
Turan, C & Yaglioglu, D, 2010. Population identification of common cuttlefish (Sepia officinalis) inferred from genetic, morphometric and cuttlebone chemistry data in the NE Mediterranean Sea. Scientia Marina, 74, 77-86.
Vidal, E.A.G., Villanueva, R., Andrade, J.P., Gleadall, I.G., Iglesias, J., Koueta, N., Rosas, C., Segawa, S., Grasse, B., Franco-Santos, R.M., Albertin, C.B., Caamal-Monsreal, C., Chimal, M.E., Edsinger-Gonzales, E., Gallardo, P., Le Pabic, C., Pascual, C., Roumbedakis, K. & Wood, J., 2014. Cephalopod Culture: Current Status of Main Biological Models and Research Priorities. In Vidal, E.A.G. (ed.) Advances in Marine Biology, 67, 1-98.
Von Boletzky, S., 1974. Effets de la sous-nutrition prolongée sur le développement de la coquille de Sepia officinalis L. (Mollusca, Cephalopoda). Bulletin de la Societe Zoologique de France, 99, 667-673.
Ward. P. D., Boletzky. S. v., 1984. Shell implosion depth and implosion morphologies in three species of Sepia (Cephalopoda) from the Mediterranean Sea. Journal of the Marine Biological Association, 64, 955-966.
Wolfram, K., Mark, F.C., John, U., Lucassen, M. & Pörtner, H.O., 2006. Microsatellite DNA variation indicates low levels of genetic differentiation among cuttlefish (Sepia officinalis L.) populations in the English Channel and the Bay of Biscay. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 1, 375-383.
Xavier, J.C., Peck, L.S., Fretwell, P. & Turner, J., 2016. Climate change and polar range expansions: could cuttlefish cross the Arctic? Marine Biology, 163 , 78.
Zaragoza, N., Quetglas, A. and Moreno, A., 2015. Identification guide for cephalopod paralarvae from the Mediterranean Sea. ICES Cooperative Research Report No. 324. pp. 91
Datasets
Bristol Regional Environmental Records Centre, 2017. BRERC species records recorded over 15 years ago. Occurrence dataset: https://doi.org/10.15468/h1ln5p accessed via GBIF.org on 2018-09-25.
Bristol Regional Environmental Records Centre, 2017. BRERC species records within last 15 years. Occurrence dataset: https://doi.org/10.15468/vntgox accessed via GBIF.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2018. Mollusc (marine) data for Great Britain and Ireland - restricted access. Occurrence dataset: https://doi.org/10.15468/4bsawx accessed via GBIF.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2023. Mollusc (marine) records for Great Britain and Ireland. Occurrence dataset: https://doi.org/10.15468/aurwcz accessed via GBIF.org on 2024-09-27.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Manx Biological Recording Partnership, 2022. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset:https://doi.org/10.15468/aru16v accessed via GBIF.org on 2024-09-27.
Merseyside BioBank., 2018. Merseyside BioBank (unverified). Occurrence dataset: https://doi.org/10.15468/iou2ld accessed via GBIF.org on 2018-10-01.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-08
Outer Hebrides Biological Recording, 2018. Invertebrates (except insects), Outer Hebrides. Occurrence dataset: https://doi.org/10.15468/hpavud accessed via GBIF.org on 2018-10-01.
South East Wales Biodiversity Records Centre, 2018. SEWBReC Molluscs (South East Wales). Occurrence dataset: https://doi.org/10.15468/jos5ga accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. Dr Mary Gillham Archive Project. Occurance dataset: http://www.sewbrec.org.uk/ accessed via NBNAtlas.org on 2018-10-02
Citation
This review can be cited as:
Last Updated: 06/11/2018