Brackish sea fir (Pachycordyle michaeli)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Nicola White | Refereed by | Dr Richard S.K. Barnes |
| Authority | (Berrill, 1948) | ||
| Other common names | - | Synonyms | Clavopsella navis (Millard, 1959), Rhizorhagium navis (Millard, 1959), Pachycordyle navis (Millard, 1959) |
Summary
Description
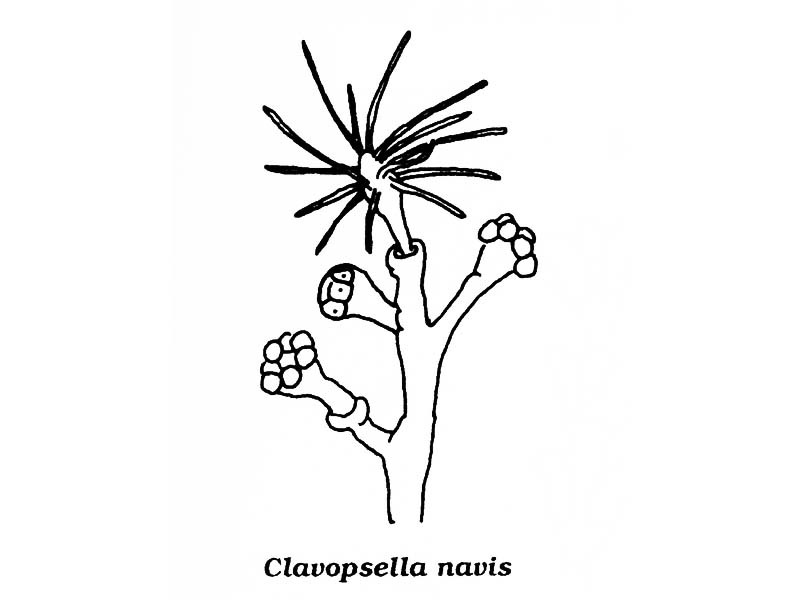
A simple hydroid consisting of an erect, unbranched stem, up to 5 mm in height, with a single terminal polyp (hydranth). Each upright stem rises from a creeping stolon (hydrorhiza). The stem is sheathed by a chitinous sheath, the perisarc. The perisarc is often wrinkled, especially near the base, and terminates below the hydranth. The hydranth bears 8 to 16 tentacles in 2 to 4 alternating whorls, depending on hydranth size. It is creamy white in colour, with hints of pink around the mouth of the hydranth. The reproductive bodies (gonophores) are borne on short stalks in an irregular spiral below the hydranth.
Recorded distribution in Britain and Ireland
Widewater lagoon, West Sussex.Global distribution
Recorded from only 3 locations worldwide: Kiel Canal, Widewater lagoon in Sussex and attached to a ship's hull in South Africa.Habitat
Grows on algae such as Chaetomorpha. It has only ever been recorded in the vicinity of ports and harbours.Depth range
-Identifying features
- Stem simple and unbranched bears a single terminal hydranth.
- Hydranth with 2-4 whorls of tentacles close to mouth.
- Gonophores in the form of fixed sporosacs.
- Planulae develop within apical part of gonophore.
Additional information
The systematic status of this species was revised by Stepanjants et al. (2000) who placed Clavopsella navis and Clavopsella quadrangularia in the new genus Thieliana. Subsequent revision by Schuchert (2004, 2007; cited in Calder, 2012) placed the species in the genus Pachycordyle. Pachycordyle navis was subsequently synonymized with Pachycordyle michaeli (WoRMS, 2021).
Listed by
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Cnidaria | Sea anemones, corals, sea firs & jellyfish |
| Class | Hydrozoa | White weeds, sea firs, sea beard and siphonophores; hydroids |
| Order | Anthoathecata | |
| Family | Bougainvilliidae | |
| Genus | Pachycordyle | |
| Authority | (Berrill, 1948) | |
| Recent Synonyms | Clavopsella navis (Millard, 1959)Rhizorhagium navis (Millard, 1959)Pachycordyle navis (Millard, 1959) | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Data deficient | ||
| Male size range | 0.39-1.29 mm | ||
| Male size at maturity | |||
| Female size range | Very small(<1cm) | ||
| Female size at maturity | |||
| Growth form | Turf | ||
| Growth rate | Data deficient | ||
| Body flexibility | High (greater than 45 degrees) | ||
| Mobility | Sessile, permanent attachment | ||
| Characteristic feeding method | Passive suspension feeder | ||
| Diet/food source | Omnivore | ||
| Typically feeds on | |||
| Sociability | |||
| Environmental position | Epifaunal | ||
| Dependency | - | ||
| Supports | - | ||
| Is the species harmful? | Data deficient | ||
Biology information
Size refers to length of hydranth.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Isolated saline water (Lagoon) |
| Biological zone preferences | Data deficient |
| Substratum / habitat preferences | Macroalgae |
| Tidal strength preferences | Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Very sheltered |
| Salinity preferences | Reduced (18-30 psu) |
| Depth range | |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
Pachycordyle navis is presumed to be an introduced species since it has only ever been recorded in the vicinity of ports and harbours. It is probably transported on ships hulls. It was first recorded in the UK in 1973 in Widewater Lagoon, Shoreham, West Sussex (Eno et al., 1997). It was last recorded there (as Clavopsella navis) by Sheader (1990) in 1990 when it was relatively abundant attached to algae. It is presumed extinct in South Africa as it has only been recorded from one ship's hull in 1959. The condition of the population in Kiel is not known.Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | |
| Fecundity (number of eggs) | 2-10 |
| Generation time | Insufficient information |
| Age at maturity | |
| Season | Insufficient information |
| Life span | Insufficient information |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | |
| Duration of larval stage | No information |
| Larval dispersal potential | No information |
| Larval settlement period |
Life history information
Female gonophores contain about 8 eggs, which develop directly into planulae. There is no free-living medusoid stage.Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidencePachycordyle navis lives attached to algae, so would be removed with the algae upon substratum loss. There would be no recovery of the population because only two extant populations of Pachycordyle navis are known: Widewater lagoon, Sussex and Kiel Canal, Germany. | High | None | Very High | Very low |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceThe species would be affected by smothering if the algae on which it lives is completely covered in the sediment. If the algae protrudes sufficiently above the sediment the hydroid may escape the effects of smothering. | Intermediate | Low | High | Very low |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidencePachycordyle navis is likely to have some tolerance to siltation as it inhabits lagoons where siltation frequently occurs. The algae on which the species lives will also lift the hydroid above the accumulation of silt. However, the heath of the host algae may be adversely affected by siltation. | Intermediate | Very low / none | High | Very low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidenceThe species is vulnerable to desiccation because it is soft bodied and has no protection from the drying effects of sun and wind. Some of the population may be sheltered from desiccation if they are present on the underside of the algal frond. However, if the whole population is destroyed recoverability would be non-existent because only two populations of Thieliana navis occur worldwide. | Intermediate | None | Very High | Very low |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceThe species is vulnerable to emergence because it is soft bodied and has no protection from desiccation. Some of the population may be sheltered from desiccation if they are present on the underside of the algal frond. However, if the whole population is destroyed recoverability would be very low because only two populations of Pachycordyle navis occur worldwide. | Intermediate | None | Very High | Very low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceThe species would probably not be affected by a change in water flow because it is permanently attached to the algae and may be able to withstand high water flow rates because they have been transported long distances on ships hulls. | Tolerant | Not relevant | Not sensitive | Very low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceThe temperature resistance of the Pachycordyle navis is not known. | No information | Not relevant | No information | Very low |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceThe species is unlikely to be affected by a change in turbidity as it is not dependant on light availability and it would not interfere with its feeding. However, the host algae may be adversely affected by a reduction in light availability. | Low | Moderate | Low | Very low |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceA change in wave exposure is unlikely to occur in a lagoon unless one of the lagoon boundaries is breached. The species would probably not be affected by an increase in wave exposure because it does not present a large surface area to wave action. However, it's host algae may be intolerant of wave exposure and may be washed away. | Tolerant | Not relevant | Not sensitive | Very low |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidenceInsufficient | No information | Not relevant | No information | Very low |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceInsufficient | No information | Not relevant | No information | Very low |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceThe species and its host algae are flexible so will 'give' under abrasion. However, they occur on top of the sediment and would probably be removed, along with surface substratum by a passing scallop dredge (or equivalent force). The impact is likely to be equivalent to substratum loss. Therefore, an intolerance of high has been recorded. | High | Low | High | Very low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidencePachycordyle navis is permanently attached to algae and would be unable to re-attach itself if removed. If the whole population is destroyed recoverability would be very low because only two populations of Pachycordyle navis occur worldwide. | High | None | Very High | Very low |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceInsufficient | No information | Not relevant | No information | Very low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Increase in salinity [Show more]Increase in salinity
EvidenceEvidence suggests that the species is tolerant of fully saline conditions because it can survive on ships hulls. The species must be tolerant of reduced salinity because it occurs in lagoons but the tolerance of the species to very reduced salinities is not known. | No information | Not relevant | No information | Not relevant |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Additional information
Importance review
Policy/legislation
| Designation | Support |
|---|---|
| Wildlife & Countryside Act | Schedule 5, section 9 |
| UK Biodiversity Action Plan Priority | Yes |
| Species of principal importance (England) | Yes |
Status
| National (GB) importance | Not rare or scarce | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | 1973 |
Importance information
-none-Bibliography
Anonymous, 1999s. Saline lagoons. Habitat Action Plan. In UK Biodiversity Group. Tranche 2 Action Plans. English Nature for the UK Biodiversity Group, Peterborough., English Nature for the UK Biodiversity Group, Peterborough.
Barnes, R.S.K., 1994. The brackish-water fauna of northwestern Europe. Cambridge: Cambridge University Press.
Boero, F., 1984. The ecology of marine hydroids and effects of environmental factors: a review. Marine Ecology, 5, 93-118.
Eno, N.C., Clark, R.A. & Sanderson, W.G. (ed.) 1997. Non-native marine species in British waters: a review and directory. Peterborough: Joint Nature Conservation Committee.
Gili, J-M. & Hughes, R.G., 1995. The ecology of marine benthic hydroids. Oceanography and Marine Biology: an Annual Review, 33, 351-426.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Millard, N.A.H., 1975. Monograph of the Hydroida of Southern Africa. Annals of the South Africa Museum, 68, 1-513.
Reise, K., Gollasch, S. & Wolff, W.J., 1999. Introduced species of the North Sea coasts. Helgoland Meeresuntersuchungen, 52, 219-234.
Sheader, M. & Sheader, A., 1990. A survey of Widewater saline lagoon to determine the current status of the site, with special reference to Ivell's sea anemone, Edwardsia ivelli. Preliminary Report, Peterborough. Nature Conservancy Council. NCC CSD Report 1176.
Sommer, C., 1992. Larval biology and dispersal of Eudendrium racemosum (Hydrozoa, Eudendriidae). Scientia Marina, 56, 205-211. [Proceedings of 2nd International Workshop of the Hydrozoan Society, Spain, September 1991. Aspects of hydrozoan biology (ed. J. Bouillon, F. Cicognia, J.M. Gili & R.G. Hughes).]
Stephanjants, S.D., Timoshkin, O.A., Anokhin, B.A. & Napara, T.A., 2000. A new species of Pachycordyle (Hydrozoa, Clavidae) from Lake Biwa (Japan), with remarks on this and related Clavid genera. Scientia Marina, 64 (Suppl. 1), 225-236.
- Calder, D.R., 2012. On a collection of hydroids (Cnidaria, Hydrozoa, Hydroidolina) from the west coast of Sweden, with a checklist of species from the region. Zootaxa, 3171, 1-77.
Datasets
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-14
Citation
This review can be cited as:
Last Updated: 24/06/2005