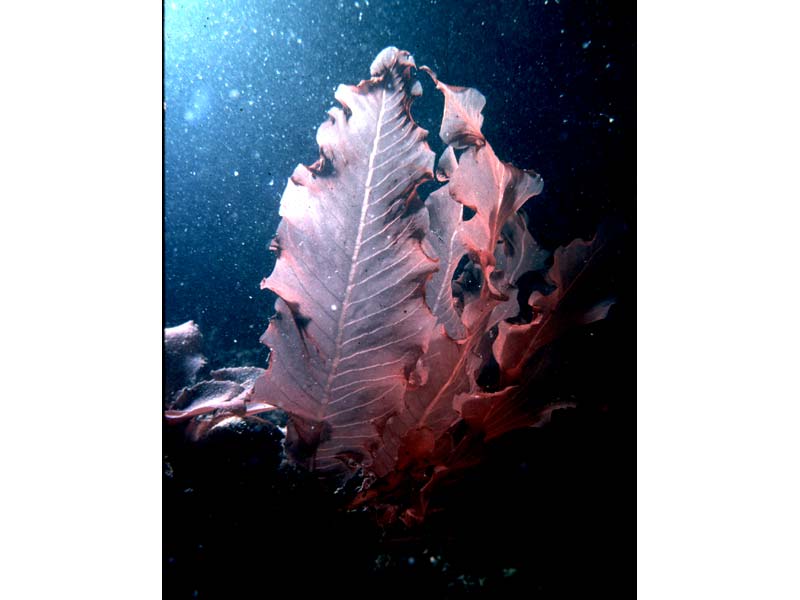
Sea beech (Delesseria sanguinea)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Dr Harvey Tyler-Walters | Refereed by | Dr Joanna Jones |
| Authority | (Hudson) J.V.Lamouroux, 1813 | ||
| Other common names | - | Synonyms | - |
Summary
Description
A conspicuous crimson seaweed up to 30 cm in length. Blades are oval or lanceolate, leaf like and reminiscent of beech leaves. The membranous lamina has a wavy margin and is supported by a conspicuous midrib with opposite pairs of lateral veins. The irregularly shaped, thickened holdfast (about 0.5 cm in diameter) gives rise to a short cylindrical stipe about 1 cm long. The stipe branches sparingly giving rise to spirally arranged blades (about 1.5 - 4 cm wide). The leaves may be pointed in young specimens. In autumn the membranous lamina is lost so that only the midrib remains. Reproductive bodies (e.g. cystocarp) develop on the naked midrib. Cystocarp are globular, with a membranous border, and form in fairly close formation on a short stalks on female plants. Carpogonia on female plants are fertilised during October but carpospores are not released until February. New fronds may grow before all reproductive structures disappear. Reproductive leaflets also grow on the denuded midrib in male and asexual plants. On the male plants tetrasporangial bladelets appear in November and tetraspores released in January and February [Kain & Bates, 1993]. Very wave battered plants may be confused with Phycodrys rubens (q.v.) which has lobed or toothed blades.
Recorded distribution in Britain and Ireland
Recorded from all coasts of the British Isles. However, records from the east coasts are sparse, presumably due to the lack of suitable substrata.Global distribution
Recorded from the north eastern coast of Iceland to the Russian coast near Murmansk. Its southern limit is in Sables d'Olonnes, northern France. It is also found in the Baltic.Habitat
May be found in deep pools in the lower eulittoral and subtidally to at least 30 m. It is a characteristic member of the understorey flora in Laminaria hyperborea (kelp) forests. It may occasionally be epiphytic on Laminaria hyperborea stipes.Depth range
1-30mIdentifying features
- Bright red, often crimson in colour.
- Margin of lamina wavy but not serrated.
- Similar to beech leaves in appearance.
- Midrib and blade veins conspicuous.
- Usually branched from, or near to, the base of the stipe.
Additional information
Young specimens may be confused with Apoglossum ruscifolium (q.v.) or Hypoglossum hypoglossoides (q.v.) although these species lack the conspicuous lateral veins of Delesseria sanguinea. Wave eroded (battered) specimens may resemble Phycodrys rubens. However, true Phycodrys rubens has lobed or toothed blades and reproductive structures are born on mature blades.
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Rhodophyta | Red seaweeds |
| Class | Florideophyceae | |
| Order | Ceramiales | |
| Family | Delesseriaceae | |
| Genus | Delesseria | |
| Authority | (Hudson) J.V.Lamouroux, 1813 | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Moderate density | ||
| Male size range | |||
| Male size at maturity | |||
| Female size range | Medium-large(21-50cm) | ||
| Female size at maturity | |||
| Growth form | Turf | ||
| Growth rate | Insufficient information | ||
| Body flexibility | |||
| Mobility | |||
| Characteristic feeding method | Autotroph | ||
| Diet/food source | |||
| Typically feeds on | Not relevant | ||
| Sociability | |||
| Environmental position | Epifloral | ||
| Dependency | Independent. | ||
| Supports | No information found | ||
| Is the species harmful? | No | ||
Biology information
Delesseria sanguinea is perennial and exhibits a complex life cycle. This species exhibits a strong seasonal pattern of growth and reproduction. New blades appear in February and grow to full size by May -June becoming increasing battered or torn and the lamina are reduced to midribs by December (Maggs & Hommersand, 1993). Blade weight is maximal in midsummer, growth dropping in June and July and becoming zero in August (Kain, 1984). Small new blades may be formed in darkness, reserves translocated from assimilates stored in the frond ribs and stipes which persist in winter (Luning, 1990; Maggs & Hommersand, 1993). Kain (1987) suggested that new blade growth may result from an increase in irradiance and hence inhibition of reproduction (e.g. due to removal of Laminarian plants from a kelp canopy) which may explain occasional crop of new blades noted in summer. Kain (1987) also suggested that the normal seasonal trigger for new blade production was temperature, probably when temperatures fell to 13 deg C or below. Morphology, salinity and temperature tolerances differ between North Sea and Baltic populations. In the Baltic specimens are smaller than British specimens, with thinner blades. Temperature and salinity tolerances are probably genetically determined (Rietema, 1993).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Strait or Sound, Ria or Voe, Enclosed coast or Embayment |
| Biological zone preferences | Lower eulittoral, Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Bedrock, Large to very large boulders, Rockpools, Small boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Exposed, Extremely exposed, Moderately exposed, Very exposed |
| Salinity preferences | Full (30-40 psu), Reduced (18-30 psu), Variable (18-40 psu) |
| Depth range | 1-30m |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
-
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Oogamous |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | >1,000,000 |
| Generation time | Insufficient information |
| Age at maturity | Insufficient information |
| Season | October - December |
| Life span | 5-10 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Spores (sexual / asexual) |
| Duration of larval stage | Not relevant |
| Larval dispersal potential | No information |
| Larval settlement period | Not relevant |
Life history information
Dickinson (1963) suggested a lifespan of 5-6 years but Kain (1984) estimated that 1 in 20 specimens may attain 9 to 16 years of age. All reproductive structures in Delesseria sanguinea are born on the mibribs. The typical life cycle of members of the Ceramiales is summarised as follows:
- Male haploid gametophytes release male gametes (spermatia) from spermatangia on male bladelets.
- Female haploid gametophytes produce the female gamete, the carpogonium on female bladelets
- After fusion (fertilization) the carposporophyte develops, enclosed in a stalked cystocarp and releases diploid carpospores.
- Carpospores develop into the tetrasporophyte, a diploid sporophyte stage.
- The sporophyte develops tetrasporangia in which haploid tetraspores are formed by meiosis.
- The tetraspores develop into gametophytes.
The gametophyte and sporophyte stages in the order Ceramiales are isomorphic (Bold & Wynne, 1978). The onset of sexual reproduction is stimulated by day length, Delesseria sanguinea is a short-day plant sensitive to a night break (Kain, 1991; Kain, 1996]. The male bladelets and spermatangia develop between September and December in the Isle of Man. (Kain, 1993). Cystocarps and tetrasporangia appear from December to March and the carpospores and tetraspores are first released in December. Female carpogonia develop 2-3 months before the carposporophytes (c. September). Tetrasporangia form in response to shorter day length (<10 hour days) than male and female gametangia (Kain, 1996). In culture male bladelets were stimulated by 11-12h days, spermatangia taking 4 weeks to develop. Spermatangia were inhibited by increased day length in culture. Kain (1987) suggested that the southern limit of Delesseria sanguinea may be determined by winter temperatures. Studies in Roscoff and Helgoland showed similar seasonality; new blades formed in April to June at Roscoff, males plants in October to December, cystocarps and tetrasporangia in October to December, the last cystocarps found in April. Juvenile recruitment occurred between February and April or June in both Roscoff and Helgoland (Molenaar & Breeman, 1997).
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceDelesseria sanguinea will be removed with the substratum and it is therefore highly intolerant. There is little information on recruitment or recolonization rates. Kain (1975) examined recolonization of cleared concrete blocks in a subtidal kelp forest. Red algae colonized blocks within 26 weeks in the shallow subtidal (0.8 m) and 33 weeks at 4.4 m. Delesseria sanguinea was noted within 41 weeks (8 months) at 4.4m in one group of blocks and within 56-59 days after block clearance in another group of blocks. This recolonization occurred during winter months following spore release and settlement, but not in subsequent samples (Kain, 1975). This suggests that recolonization of Delesseria sanguinea in new areas is directly dependent on spore availability. Rhodophyceae have non flagellate, and non-motile spores that stick on contact with the substratum. Norton (1992) noted that algal spore dispersal is probably determined by currents and turbulent deposition. However, red algae produce large numbers of spores that may settle close to the adult especially where currents are reduced by an algal turf or in kelp forests. It is likely that this species could recolonize an area from adjacent populations within a short period of time in ideal conditions but that recolonization from distant populations would probably take longer. | High | High | Moderate | Moderate |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceAdults are up to 30 cm in height and will probably survive smothering to a depth of 5 cm by sediment. However, algal spores and propagules are adversely affected by a layer of sediment, which can exclude up to 98 percent of light (Vadas et al., 1992). Germlings and juveniles are likely to be highly intolerant of smothering. A layer of sediment is likely to interfere with settlement and attachment of spores. | Intermediate | High | Low | Moderate |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceThe effects of increased siltation on adults is likely to include smothering (above) or increase turbidity and therefore light attenuation (see below). Within kelp forest, current flows are reduced and siltation is likely to be increased. Therefore, Delesseria sanguinea may be tolerant of a level of siltation. Increased siltation may increase sediment scour, especially in winter. Spores, germlings and juveniles are likely to be highly intolerant of sediment scour (Vadas et al., 1992). However, Delesseria sanguinea reproduces in winter and increased siltation may interfere with recruitment and long term survival of the population. | Low | Immediate | Not sensitive | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidenceDelesseria sanguinea is normally subtidal and unlikely to be exposed to desiccation except at extreme low tides or in exposed rock pools. Strong insolation of 20mW per square cm (comparable to mid day on a cloudless December in the UK) causes significant reduction in photosynthesis in only a few hours (1- 4 hrs) (Drew, 1983). A variety of subtidal red algae can survive aerial exposure for 14 hrs only at humidities of 100 percent (Kain & Norton, 1990). Spores and germlings are highly intolerant of desiccation due to there relatively high surface to area ratios. Subtidal algae are more intolerant of than intertidal algae to desiccation. | High | High | Moderate | Low |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceDelesseria sanguinea is a subtidal species. Increased emergence will extend tidal influence further down the shore. The extent of the population will be decreased accordingly by depressing its height up the shore as a result of increased desiccation, insolation and competition from intertidal algae. Similarly individuals inhabiting rock pools may be lost. | Intermediate | High | Low | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceAlgae are dependent on water flow for a supply of nutrients and removal of wastes. Delesseria sanguinea is found is a wide range of water flow regimes from moderately strong to weak. However, the flow rates experienced within kelp forests will be reduced. Deep growing red algae such as Delesseria sanguinea were observed growing in stagnating water in Kiel Bay, western Baltic Sea (Schwenke, 1960; cited in Kinne, 1971). It is likely, therefore, that this species would tolerate decreased water flow. However, an increase to strong or very strong may inhibit settlement of spores and may remove adults or germlings. | Intermediate | High | Low | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceThere is some evidence to suggest that blade growth in Delesseria sanguinea is delayed until ambient sea temperatures fall below 13 °C, although blade growth is likely to be intrinsically linked to gametangia development (see Kain, 1987). Delesseria sanguinea is tolerant of 23 deg C for a week (Lüning, 1984) but dies rapidly at 25 deg C. North Sea and Baltic specimens grew between 0-20 deg C, survived at 23 deg C but died at 25 deg C rapidly (Rietema, 1993). Rietema (1993) reported temperature differences in temperature tolerance between North Sea and Baltic specimens. Lüning (1990) reports optimal growth in Delesseria sanguinea between 10 - 15 deg C and optimal photosynthesis at 20 deg C. However, the upper limit of temperature tolerance is reduced by lowered salinity in Baltic specimens (Kinne, 1970; Kain & Norton, 1990). At low salinity photosynthesis is restricted to a narrow range of temperatures in adult thalli whereas juvenile thalli have a wider response range (Lobban & Harrison, 1997; fig 6.27). It is likely therefore that within the subtidal an increase in temperature of 2 deg C in the long term will have limited effect on survival, although it may affect initiation of new growth at the southern limits of the population. An increase of 5 deg C in the short term may affect survival if the ambient temperature is increased above 23 deg C. | Low | Immediate | Not sensitive | Moderate |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceTemperate, sub-tidal red algae are characterised by high rates of photosynthetic saturation, high maximum rates of photosynthesis and rapid growth. Reproduction in some species, triggered by short days, during winter when growth of other algae is limited, increased wave action results in loss of algae and most space is available for recolonization (Kain & Norton, 1990). Red algae possess only trace amounts of chlorophyll a (Lüning & Schmitz, 1988). Their main photosynthetic pigments are phycobiliproteins, which absorb optimally in the green light of deeper coastal waters. Photosynthesis in Delesseria sanguinea is inhibited by high light levels >200 µmol/m²/s, roughly equivalent to very clear shallow water in summer (Kain & Norton, 1990; Figure 15-2). However, in turbid coastal waters, where green light prevails, photosynthetic effectiveness increases with depth in red algae rich in phycoerythrin such as Delesseria sanguinea (Lüning, 1990). Delesseria sanguinea can grow in darkness using energy reserves stored in the stipe or lower regions of the frond ribs (Lüning, 1990). Increased turbidity would decrease the light levels at depth and may reduce the effective day length and induce reproduction earlier than in less turbid areas (Kain & Norton, 1990). Delesseria sanguinea is adapted to grow at depth or in the shade of other plants. Long term (years) decreased turbidity may restrict its downward extent. Short term changes may affect growth and reproduction, however, as a perennial, the adults will probably survive. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceDelesseria sanguinea occurs on coasts with a wide range of wave exposures, from very exposed to very sheltered and are therefore unlikely to be intolerant of changes in wave action. The plants are sheltered from the worst effects of wave action by depth or dominant kelps. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidencePlants have no known sound or vibration receptors | Tolerant | Not relevant | Not sensitive | Not relevant |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceMarine algae have no known response to visual stimuli. | Tolerant | Not relevant | Not sensitive | Not relevant |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceLittle information is available on the effects of abrasion on sub tidal red algae. However, the growth form of Delesseria sanguinea suggests that its lamina would probably be damaged by passing fishing gear, such as a scallop dredge (see benchmark). Although, its lamina is flexible a proportion of the population is likely to be torn off and lost. Specimens attached to cobbles or boulders may be removed (see substratum loss above). Therefore, an intolerance of intermediate has been recorded. | Intermediate | High | Low | Very low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceIf Delesseria sanguinea is removed from the substratum it can not reattach itself and it is therefore highly intolerant. Kain (1975) examined recolonization of cleared concrete blocks in a subtidal kelp forest. Red algae colonized blocks within 26 weeks in the shallow subtidal (0.8 m) and 33 weeks at 4.4 m. Delesseria sanguinea was noted within 41 weeks (8 months) at 4.4 m in one group of blocks and within 56-59 days in blocks cleared at two monthly intervals during winter months, but not in subsequent samples (Kain, 1975). This suggests that Delesseria sanguinea can recolonize areas, but is directly dependent on its reproductive season and spore availability. Rhodophyceae have non flagellate, and non-motile spores that stick on contact with the substratum. Norton (1992) noted that algal spore dispersal is probably determined by currents and turbulent deposition. However, red algae produce large numbers of spores that may settle close to the adult especially where currents are reduced by an algal turf or in kelp forests. It is likely that this species could recolonize an area from adjacent populations within a short period of time in ideal conditions but that recolonization from distant populations would probably take longer. | High | High | Moderate | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceO'Brien & Dixon (1976) suggested that red algae were the most sensitive group of algae to oil or dispersant contamination, possibly due to the susceptibility of phycoerythrins to destruction, and that the filamentous forms were the most sensitive. However, most evidence relates to dispersants. O'Brien & Dixon (1976) also report that red algae are effective indicators of detergent damage since they undergo colour changes when exposed to relatively low concentration of detergent. Smith (1968) reported that 10 ppm of the detergent BP 1002 killed the majority of specimens in 24hrs in toxicity tests. However, the effects take several days to manifest; when killed the lamina turn bright orange. Heavy mortality of Delesseria sanguinea occurred down to 12 m after the 'Torrey Canyon' oil spill (probably due to a mixture of wave action and dispersant application). Laboratory studies of the effects of oil and dispersants on several red algae species, including Delesseria sanguinea (Grandy, 1984; cited in Holt et al., 1995) concluded that they were all sensitive to oil/ dispersant mixtures, with little differences between adults, sporelings, diploid or haploid life stages. Cole et al. (1999) suggested that herbicides , such as simazina and atrazine were very toxic to macrophytes. Hoare & Hiscock (1974) noted that Delesseria sanguinea was excluded from Amlwch Bay, Anglesey by acidified halogenated effluent discharge. Holt et al. (1995) concluded that Delesseria sanguinea is probably generally sensitive to chemical contamination. | High | High | Moderate | High |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceBryan (1984) suggested that the general order for heavy metal toxicity in seaweeds is: Organic Hg > inorganic Hg > Cu > Ag > Zn > Cd > Pb. Cole et al. (1999) reported that Hg was very toxic to macrophytes. The sub-lethal effects of Hg (organic and inorganic) on Plumaria elegans sporeling were reported by Boney (1971), for example 100 percent growth inhibition was caused by 1 ppm Hg in his study. However, little information concerning the effects of heavy metals on Delesseria sanguinea was found. Heavy metals have the potential to accumulate in plant tissue, therefore it may take some time for tissue levels to fall before recovery can begin. | Low | Very high | Very Low | Low |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceDelesseria sanguinea is unlikely to become smothered by oil due to its subtidal position. However, O'Brien & Dixon (1976) suggested that red algae were the most sensitive group to oil or dispersant contamination, possibly due to the susceptibility of phycoerythrins to destruction, and that the filamentous forms were the most intolerant. Heavy mortality of Delesseria sanguinea occurred down to 12 m after the 'Torrey Canyon' oil spill, although it was unclear how much of the effect was due to oil rather than dispersant contamination. Laboratory studies of the effects of oil and dispersants on several red algae species, including Delesseria sanguinea (Grandy, 1984; cited in Holt et al., 1995) concluded that they were all sensitive to oil/ dispersant mixtures, with little differences between adults, sporelings, diploid or haploid life stages. Holt et al. (1995) concluded that Delesseria sanguinea is probably generally sensitive to chemical contamination. | High | High | Moderate | High |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceKain (1984) noted that growth of blades continued into July and August. Delesseria sanguinea can grow new blades in darkness by drawing on reserves held in frond midrib and stipe (Lüning, 1990), suggesting that nutrients are subject to 'luxury' accumulation in the winter months. Delesseria sanguinea is likely to tolerate low nutrient levels, during spring and summer. An increase in abundance of red algae, including Delesseria sanguinea, was associated with eutrophication in the Skagerrak area, Sweden, especially in areas with the most wave exposure or water exchange (Johansson et al., 1998). However, where eutrophication resulted in high siltation rates, the delicate foliose red algae such as Delesseria sanguinea were replaced by tougher, erect red algae (Johansson et al., 1998). High nutrient levels and eutrophication may result in increased siltation and turbidity (see above). Although increased nutrients may stimulate growth, this species may be out competed by green algae and epiphytes at the upper reaches of its range where light is less limiting. | Low | Immediate | Not sensitive | Low |
Increase in salinity [Show more]Increase in salinity
EvidenceSalinity and temperature affect photosynthesis. At low salinities photosynthesis in adults occurs in a restricted temperature range, although juvenile thalli photosynthesise across a wider range of temperatures (Lehnberg, 1978; cited in Lobban & Harrison, 1997). Rietema (1993) examined ecotypic differences between North Sea and Baltic populations of Delesseria sanguinea. Optimal growth occurred in Baltic specimens at 19 -23 psu and North Sea specimens at 33 psu. North Sea specimens died at 7.5 - 11 psu. Optimal photosynthesis occurred at full salinity, even in specimens collected from 15 psu (Lehnberg, 1978; cited in Lobban & Harrison, 1997). Increased salinity at 40 psu drastically reduced photosynthesis in Baltic specimens (Kinne, 1971). Delesseria sanguinea is likely to tolerate reduced salinity, although growth and reproduction may be reduced in low salinity environments when compared to full salinity. | Low | Immediate | Not sensitive | High |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceThe effects of deoxygenation in plants has been little studied. Since plants produce oxygen they may be considered relatively insensitive. However, a study of the effects of anaerobiosis (no oxygen) on some marine algae concluded that Delesseria sanguinea was very intolerant of anaerobic conditions; at 15 deg C death occurs within 24hrs and no recovery takes place although specimens survived at 5 deg C. (Hammer, 1972). | High | High | Moderate | Moderate |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceNo reference to diseases of red algae was found in the literature. | No information | No information | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceNo non-native species were identified that compete with Delesseria sanguinea. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceThis species has been collected, by the French company GoËmar for cosmetic purposes, although knowledge of this trend continuing is unknown. Additionally sheep, on North Ronaldsay of the Orkneys have been known to graze on this species of algae. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceExtraction or harvesting of kelp will increased light penetration and is likely to enhance growth. Kain (1975) showed that red algae recolonized cleared concrete blocks, in kelp forest in the Isle of Man; Delesseria sanguinea colonizing within 56-59 days. This suggests that extraction of kelp may encourage growth of this species in the short term until the kelp species dominate again. | Tolerant* | Not relevant | Not sensitive* | Low |
Additional information
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Delesseria sanguinea is used in the cosmetics industry for its anticoagulant properties and vitamin K content; the active principle being termed delesserine (Guiry & Blunden, 1991).Bibliography
Bold, H.C. & Wynne, M.J., 1978. Introduction to the algae: structure and reproduction. New-Jersey: Prentice-Hall, Inc.
Boney, A.D., 1971. Sub-lethal effects of mercury on marine algae. Marine Pollution Bulletin, 2, 69-71.
Dickinson, C.I., 1963. British seaweeds. London & Frome: Butler & Tanner Ltd.
Drew, E.A., 1983. Light. In Sublittoral ecology: The ecology of the shallow sublittoral benthos, (ed. R. Earll & D.G. Erwin). Oxford: Clarendon Press.
Guiry, M.D. & Blunden, G., 1991. Seaweed Resources in Europe: Uses and Potential. Chicester: John Wiley & Sons.
Hammer, L., 1972. Anaerobiosis in marine algae and marine phanerograms. In Proceedings of the Seventh International Seaweed Symposium, Sapporo, Japan, August 8-12, 1971 (ed. K. Nisizawa, S. Arasaki, Chihara, M., Hirose, H., Nakamura V., Tsuchiya, Y.), pp. 414-419. Tokyo: Tokyo University Press.
Hardy, F.G. & Guiry, M.D., 2003. A check-list and atlas of the seaweeds of Britain and Ireland. London: British Phycological Society
Hiscock, S., 1986b. A field key to the British Red Seaweeds. Taunton: Field Studies Council. [Occasional Publication No.13]
Hoare, R. & Hiscock, K., 1974. An ecological survey of the rocky coast adjacent to the effluent of a bromine extraction plant. Estuarine and Coastal Marine Science, 2 (4), 329-348.
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Johansson ,G., Eriksson, B.K., Pedersen, M. & Snoeijs, P., 1998. Long term changes of macroalgal vegetation in the Skagerrak area. Hydrobiologia, 385, 121-138.
Kain, J.M., & Bates, M.J., 1993. The reproductive phenology of Delesseria sanguinea and Odonthalia dentata off the Isle of Man. European Journal of Phycology, 28, 173-182
Kain, J.M., & Norton, T.A., 1990. Marine Ecology. In Biology of the Red Algae, (ed. K.M. Cole & Sheath, R.G.). Cambridge: Cambridge University Press.
Kain, J.M., 1975a. Algal recolonization of some cleared subtidal areas. Journal of Ecology, 63, 739-765.
Kain, J.M., 1982. The reproductive phenology of nine species of the Rhodophycota in the subtidal region of the Isle of Man. British Phycological Journal, 17, 321-331.
Kain, J.M., 1987. Photoperiod and temperature as triggers in the seasonality of Delesseria sanguinea. Helgolander Meeresuntersuchungen, 41, 355-370.
Kain, J.M., 1991. The dithering males of Delesseria. British Phycological Journal, 26, 90.
Kain, J.M., 1996. Photoperiodism in Delesseria sanguinea (Ceramiales, Rhodophyta) 1. The phases and sexes differ. Phycologia, 35, 446-455.
Kinne, O. (ed.), 1970. Marine Ecology: A Comprehensive Treatise on Life in Oceans and Coastal Waters. Vol. 1 Environmental Factors Part 1. Chichester: John Wiley & Sons
Kinne, O. (ed.), 1971a. Marine Ecology: A Comprehensive, Integrated Treatise on Life in Oceans and Coastal Waters. Vol. 1 Environmental Factors, Part 2. Chichester: John Wiley & Sons.
Kühl, H., 1981. The sand gaper Mya arenaria. In Invertebrates of the Wadden Sea. Final report of the section 'Marine Zoology' of the Wadden Sea Working Group, (ed. N. Dankers, H. Kuhl, W.J., Wolff), pp. 118-119.
Lobban, C.S. & Harrison, P.J., 1997. Seaweed ecology and physiology. Cambridge: Cambridge University Press.
Lüning, K., & Schmitz, K., 1988. Dark growth of the red algae Delesseria sanguinea, (Cerimales): lack of chlorophyll, photosynthetic capability, and phycobilisomes. Phycologia,27, 72-77
Lüning, K., 1984. Temperature tolerance and biogeography of seaweeds: the marine algal flora of Helgoland (North Sea) as an example. Helgolander Meeresuntersuchungen, 38, 305-317.
Maggs, C.A. & Hommersand, M.H., 1993. Seaweeds of the British Isles: Volume 1 Rhodophycota Part 3A Ceramiales. London: Natural History Museum, Her Majesty's Stationary Office.
Molenaar, F.J. & Breeman, A.M., 1997. Latitudinal trends in the growth and reproductive seasonality of Delesseria sanguinea, Membranoptera alata, and Phycodrys rubens (Rhodophyta). Journal of Phycology, 33, 330-343.
Norton, T.A. (ed.), 1985. Provisional Atlas of the Marine Algae of Britain and Ireland. Huntingdon: Biological Records Centre, Institute of Terrestrial Ecology.
Norton, T.A., 1992. Dispersal by macroalgae. British Phycological Journal, 27, 293-301.
Picton, B.E. & Costello, M.J., 1998. BioMar biotope viewer: a guide to marine habitats, fauna and flora of Britain and Ireland. [CD-ROM] Environmental Sciences Unit, Trinity College, Dublin.
Rietema, H., 1993. Ecotypic differences between Baltic and North Sea populations of Delesseria sanguinea and Membranoptera alata. Botanica Marina, 36, 15-21.
Smith, J.E. (ed.), 1968. 'Torrey Canyon'. Pollution and marine life. Cambridge: Cambridge University Press.
South, G.R. & Tittley, I., 1986. A Checklist and Distributional Index of the Benthic Marine Algae of the North Atlantic Ocean. London: British Museum (Natural History).
Vadas, R.L., Johnson, S. & Norton, T.A., 1992. Recruitment and mortality of early post-settlement stages of benthic algae. British Phycological Journal, 27, 331-351.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2014. Occurrence dataset: https://doi.org/10.15468/erweal accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2016. Occurrence dataset: https://doi.org/10.15468/146yiz accessed via GBIF.org on 2018-09-27.
Kent Wildlife Trust, 2018. Biological survey of the intertidal chalk reefs between Folkestone Warren and Kingsdown, Kent 2009-2011. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Manx Biological Recording Partnership, 2017. Isle of Man wildlife records from 01/01/2000 to 13/02/2017. Occurrence dataset: https://doi.org/10.15468/mopwow accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2022. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset:https://doi.org/10.15468/aru16v accessed via GBIF.org on 2024-09-27.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-08
Outer Hebrides Biological Recording, 2018. Non-vascular Plants, Outer Hebrides. Occurrence dataset: https://doi.org/10.15468/goidos accessed via GBIF.org on 2018-10-01.
Royal Botanic Garden Edinburgh, 2018. Royal Botanic Garden Edinburgh Herbarium (E). Occurrence dataset: https://doi.org/10.15468/ypoair accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. SEWBReC Algae and allied species (South East Wales). Occurrence dataset: https://doi.org/10.15468/55albd accessed via GBIF.org on 2018-10-02.
Yorkshire Wildlife Trust, 2018. Yorkshire Wildlife Trust Shoresearch. Occurrence dataset: https://doi.org/10.15468/1nw3ch accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 09/11/2006