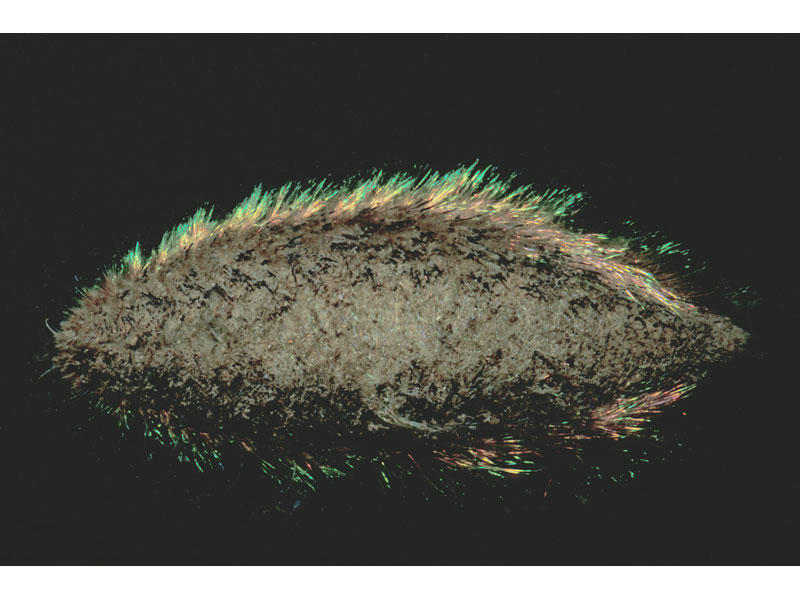
Sea mouse (Aphrodita aculeata)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Dr Harvey Tyler-Walters & Joelene Hughes | Refereed by | This information is not refereed |
| Authority | Linnaeus, 1758 | ||
| Other common names | - | Synonyms | Aphrodite aculeta |
Summary
Description
An oval bodied worm of around 10-20 cm long with a width of up to 6 cm. This polychaete has a distinctive covering of chaeta and bristles giving it a mat-like, 'felt' appearance. Some chaetae are iridescent giving the flanks a blue, green, yellow and bronze shimmer. The ventral underside is yellow/brown in colour and forms a ridged, flattened sole. The head is hidden but two horn-like palps protrude in front.
Recorded distribution in Britain and Ireland
Found around the coasts of Britain and Ireland.Global distribution
Reported from the North Atlantic including off Newfoundland and the North Sea, the Baltic, and the Mediterranean.Habitat
Found in the sublittoral to depths of over 1000 m in muddy sand.Depth range
Sub-littoral to over 1000 mIdentifying features
- A polychaete worm composed of around 40 segments covered with 15 pairs of elytra (scales).
- Dorsal surface is convex and completely covered in a dense mat of long, fine chaetae.
- A fringe is formed along the lateral lines by thick dark coloured bristles mixed with longer green, blue and gold iridescent cirri.
- The chaetae cover 15 pairs of smooth elytra.
- The long cirri project through from segments not bearing elytra.
- The ventral surface is a brownish yellow colour, flattened and heavily papillate.
- The concealed head has two pairs of sessile eyes either side of a single antenna.
- Two palps and two pairs of shorter tentacles protrude from the head.
Additional information
Aphrodita aculeata is named after the Greek goddess of love. All members of the family Aphroditidae are characterized by scales (the elytra) on their back (dorsal surface) which, in Aphrodita aculeata, are covered by a conspicuous layer of long, fine chaetae forming a mat of 'felt'. Detailed descriptions of this species are given by Fordham (1925), Chamber & Muir (1997) and Barnich & Fiege (2000). Aphrodita aculeata is distinguished from Aphrodita alta and Aphrodita perarmata by the presence of iridescent lateral chaetae in Aphrodita aculeata (Barnich & Fiege, 2000). Individuals may be found washed up on shores after storms or stranded during low tides.
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Annelida | Segmented worms e.g. ragworms, tubeworms, fanworms and spoon worms |
| Class | Polychaeta | Bristleworms, e.g. ragworms, scaleworms, paddleworms, fanworms, tubeworms and spoon worms |
| Order | Phyllodocida | |
| Family | Aphroditidae | |
| Genus | Aphrodita | |
| Authority | Linnaeus, 1758 | |
| Recent Synonyms | Aphrodite aculeta | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Low density | ||
| Male size range | 10-20 cm | ||
| Male size at maturity | |||
| Female size range | Medium(11-20 cm) | ||
| Female size at maturity | |||
| Growth form | Vermiform segmented | ||
| Growth rate | Data deficient | ||
| Body flexibility | High (greater than 45 degrees) | ||
| Mobility | Swimmer (appendages, paddles), Burrower | ||
| Characteristic feeding method | Active suspension feeder, Predator | ||
| Diet/food source | Carnivore | ||
| Typically feeds on | Other polychaetes (see additional information below). | ||
| Sociability | No information | ||
| Environmental position | Infaunal | ||
| Dependency | Independent. | ||
| Supports | Substratum species of entoproct (see additional information). | ||
| Is the species harmful? | No | ||
Biology information
Little information on the biology of this species was found. However, a detailed description of its anatomy is given by Fordham (1925).
Feeding. Mettam (1980) found that Aphrodita aculeata was an active predator feeding primarily on other worms, including both large active polychaetes and sedentary polychaetes. For example, the gut contents of Aphrodita aculeata were reported to contain the remains of Pectinaria and Lumbriconereis; polynoids, nereids, sabellids and terebellid polychaetes; nemerteans, and very young crabs and hermit crabs. In laboratory experiments, Aphrodita aculeata did not feed unless buried and only attacked prey overnight. In the laboratory, it fed on Nephtys hombergi, Hediste diversicolor and Nereis virens. Prey was swallowed whole, head first, passing slowly into the intestine, and its remains were deposited in a faecal pellet in the same order, i.e. head first (Mettam, 1980). Swallowing large prey is a laboured process (Mettam, 1980), e.g. the king rag Nereis virens, is about three times the length of the sea mouse. The swallowing of Nereis virens by the sea mouse was likened "to a hedgehog swallowing a snake" (Gunnar Thorson pers comm. cited in Mettam, 1980).
Movement. Mettam (1971) suggested that the wide body shape of Aphrodite aculeata was an adaptation to the 'slow crawling' mechanism of locomotion found in other polychaetes. Forward propulsion is achieved by the movement of individual parapodia in a 'fast stepping pattern' rather than the sinusoidal undulations characteristic of many other polychaete worms. For an illustration and detail of the musculature and mechanism involved see Mettam (1971).
Commensals. Aphroditoidea are known to harbour a variety of organisms under their scales and chaetae. Aphrodita aculeata was reported to host several entoprocts, e.g. Loxosomella claviformis, Loxosomella fauveli and Loxosomella obesa (Chambers & Muir, 1997).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast, Offshore seabed, Strait or Sound, Sea loch or Sea lough, Enclosed coast or Embayment |
| Biological zone preferences | Lower circalittoral, Lower infralittoral, Upper circalittoral, Upper infralittoral |
| Substratum / habitat preferences | Coarse clean sand, Fine clean sand, Mixed, Mud, Muddy gravel, Muddy sand, Sandy mud |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Exposed, Extremely sheltered, Moderately exposed, Sheltered, Very sheltered |
| Salinity preferences | Full (30-40 psu) |
| Depth range | Sub-littoral to over 1000 m |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
Barnich & Fiege (2000) cite a record of Aphrodita aculeata from a depth of 3000 m in the Atlantic.Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | |
| Reproductive frequency | No information |
| Fecundity (number of eggs) | No information |
| Generation time | Insufficient information |
| Age at maturity | Insufficient information |
| Season | See additional information |
| Life span | Insufficient information |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Lecithotrophic |
| Duration of larval stage | No information |
| Larval dispersal potential | No information |
| Larval settlement period | Insufficient information |
Life history information
Little information on the reproduction and development of this species was found.
Gametogenesis. Aphrodita aculeata is dioecious, i.e. has separate sexes. In females, the ova (eggs), and in males the sperm, develop from the peritoneal sheath of the blood vessels (except the major dorsal and ventral vessels and branches close to the intestine) (Fordham, 1925). The ova and sperm are released into the body cavity (coelum) where the sperm complete their development. Mature females can be identified by 'cream-coloured' eggs visible through the thin walls of the parapodia. In mature males, the coelum is filled with a milky fluid, i.e. sperm (Fordham, 1925). No spermatophore was observed, although sperm may be arranged in groups of up to four (Fordham, 1925). Presumably, large numbers of eggs and sperm are released although no estimate of fecundity was found.
Spawning. Sperm and ova are shed through the nephridia (the annelid excretory organs) and their nephridiopores on the dorsal surface (Fordham, 1925). Mature males and females were observed at Plymouth in October when males were seen to spawn, although mature specimens were also collected in March (Fordham, 1925). Fordham (1925) also reported mature individuals in May and spawning in June (location unknown), and mature females in the Naples area in September. Individuals were observed spawning off Rame, Plymouth in November 1923 and mature females were collected in the Plymouth area in September 1930 (MBA, 1957). Thorson (1946) reports spawning in the Naples area in January and February, in aquaria in Naples in March, and mature females in the St Andrews area in May. Overall, Thorson (1946) suggested that spawning occurred in winter and spring.
Larval development. Larval development is probably but not necessarily similar to related species of Aphroditidae such as Hermonia hystrix and to a lesser degree to members of the Polynoidae such as Harmothoe lunulata (as imbricata). Larval development is lecithotrophic in the Aphroditidae so far studied i.e. Hermonia hystrix (Rouse & Pleijel, 2001). The larva is probably a ciliated, free-swimming trochophore, which develops into a juvenile composed of only a few segments on settlement. The larvae of Hermonia hystrix has a long pelagic phase (von Draschke, 1885 cited in Thorson, 1946). However, although Aphrodite aculeata were very common in the Øresund and, therefore, their larvae were expected to be common in the plankton, none were found in a four-year period (Thorson, 1946). Therefore, Thorson (1946) suggested that the larvae of Aphrodite aculeata either had a very short pelagic phase or non-pelagic development. However, no information on the larval development of this species was found.
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceLoss of its substratum would result in loss of the resident population and an intolerance of high has been recorded. Once the habitat has returned to suitable conditions, recovery may be high (see additional information below). | High | High | Moderate | Low |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceAphrodita aculeata is an active burrowing species living in the surface sediment. Deposition of 5 cm of similar sediment is unlikely to adversely affect the species directly. Deposition of sediment that differs from that present is likely to modify the sediment structure, which may affect this species, depending on the nature of the change. However, at the benchmark level an intolerance of low has been recorded. Recovery is likely to be rapid. | Low | Immediate | Not sensitive | Low |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceAphrodita aculeata is an active predator and unlikely to be directly affected by changes in suspended sediment levels. It preys predominantly on other worms (Mettam, 1980) some of which are likely to be suspension feeders. Suspension feeders may benefit, or be adversely affected by changes in suspended sediment levels, depending on the species. However, Aphrodita aculeata can feed on a variety of prey and would probably not be adversely affected. | Tolerant | Not relevant | Not sensitive | Not relevant |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceAphrodita aculeata is an active predator and unlikely to be directly affected by changes in suspended sediment levels. It preys predominantly on other worms (Mettam, 1980) some of which are likely to be suspension feeders. Suspension feeders may benefit, or be adversely affected by changes in suspended sediment levels, depending on the species. However, Aphrodita aculeata can feed on a variety of prey and would probably not be adversely affected. | Tolerant | Not relevant | Not sensitive | Not relevant |
Desiccation [Show more]Desiccation
EvidenceStranded Aphrodita aculeata would undoubtedly die due to desiccation and predation. However, it is normally a subtidal species unlikely to be exposed to the air. Therefore, not relevant has been recorded. | Not relevant | Not relevant | Not relevant | Not relevant |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceAn increase in emergence could potentially decrease the upper extent of the population. However, Aphrodita aculeata is a mobile species likely to migrate to deeper water. Therefore, an intolerance of low has been recorded. | Low | Very high | Very Low | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceA decrease in emergence could potentially increase the habitat available to Aphrodita aculeata, assuming suitable substratum was present. Therefore, tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Not relevant |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceChanges in water flow rate are unlikely to affect Aphrodita aculeata directly. However, water flow rate and other hydrodynamic factors have a significant effect on the distribution of sediments of different grain size in sedimentary habitats. Increased water flow deposits coarser sediments whereas reduced water flow rates will deposit finer sediments. Aphrodita aculeata has been recorded in habitats subject to very weak to moderately strong tidal streams (JNCC, 1999). An increase in water flow rate from for instance moderately strong to very strong (see benchmark) is likely to significantly alter the nature of the substratum. Therefore, an increase in water flow rate is likely to change the distribution and extent of Aphrodita aculeata populations and an intolerance of intermediate has been recorded. Once the habitat has returned to suitable conditions recovery is may be rapid (see additional information below). | Intermediate | High | Low | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceChanges in water flow rate are unlikely to affect Aphrodita aculeata directly. However, water flow rate and other hydrodynamic factors have a significant effect on the distribution of sediments of different grain size in sedimentary habitats. Increased water flow deposits coarser sediments whereas reduced water flow rates will deposit finer sediments. Aphrodita aculeata has been recorded in habitats subject to very weak to moderately strong tidal streams (JNCC, 1999). Therefore, a further decrease in water flow rate is unlikely. | Not relevant | Not relevant | Not relevant | Not relevant |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceAphrodita aculeata is a subtidal species and unlikely to be exposed to extreme temperature change due to natural events. It is also widespread, occurring off Newfoundland, in the North Sea, Baltic and the Mediterranean. Therefore, it is unlikely to be adversely affected by chronic temperature change at the benchmark level in British and Irish waters. Therefore, not sensitive has been recorded. | Tolerant | Not relevant | Not sensitive | Very low |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceAphrodita aculeata is a subtidal species and unlikely to be exposed to extreme temperature change due to natural events. It is also widespread, occurring off Newfoundland, in the North Sea, Baltic and the Mediterranean. Therefore, it is unlikely to be adversely affected by chronic temperature change at the benchmark level in British and Irish waters. Therefore, not sensitive has been recorded. | Tolerant | Not relevant | Not sensitive | |
Increase in turbidity [Show more]Increase in turbidity
EvidenceLight intensity is unlikely to affect Aphrodita aculeata directly. | Tolerant | Not relevant | Not sensitive | Low |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceLight intensity is unlikely to affect Aphrodita aculeata directly. | Tolerant | Not relevant | Not sensitive | Low |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceWave exposure and other hydrodynamic factors have a significant effect on the distribution of sediments of different grain size in sedimentary habitats, especially in shallow waters. Aphrodita aculeata has been recorded from extremely wave sheltered to wave exposed habitats (JNCC, 1999), although probably at greater depths in wave exposed locations. An increase in wave exposure from, for instance, exposed to extremely exposed, is likely to increase the erosion of fine sediments and favour coarse sediments and/or increase the mobility of the sediments. Mobile sediments are dynamic and harbour an impoverished fauna in comparison with stable sediments. Overall, an increase in wave exposure is likely to change the nature of the sediment, and reduce the extent of habitat suitable for Aphrodita aculeata. Therefore, an intolerance of intermediate has been recorded. Recoverability may be high (see additional information). | Intermediate | High | Low | Very low |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceWave exposure and other hydrodynamic factors have a significant effect on the distribution of sediments of different grain size in sedimentary habitats, especially in shallow waters. Aphrodita aculeata has been recorded from extremely wave sheltered to wave exposed habitats (JNCC, 1999), although probably at greater depths in wave exposed locations. Therefore, a decrease in wave exposure from exposed to sheltered is unlikely to have an adverse effect. | Tolerant | Not relevant | Not sensitive | Low |
Noise [Show more]Noise
EvidenceThere is little information on the effects of underwater noise or vibration on invertebrates (Vella et al., 2001). Although Aphrodita aculeata is likely to respond to the pressure wave or vibrations caused by potential predators, it is unlikely to be affected by noise at the benchmark level. | Tolerant | Not relevant | Not sensitive | Not relevant |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceMettam (1980) noted that, in the laboratory, Aphrodita aculeata only took prey overnight. This observation may suggest that it is either nocturnal or has a dislike of background lighting (as found in a laboratory). However, it probably has very poor visual acuity and is unlikely to be affected by shading or visual presence at the benchmark level. | Tolerant | Not relevant | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceLarge Aphrodita aculeata (>7 cm in length) were reported to suffer up to 31% mortality due to beam or otter trawls in sandy sediments of the North Sea (Bergman & van Santbrink, 2000). No mortality was reported in smaller individuals, presumably because they could pass through the trawls unharmed. Bergman & van Santbrink (2000) also reported an annual mortality for Aphrodita aculeata of up to 20% due to trawling in the Dutch sector of the North Sea in 1994. Kaiser et al. (1998) examined the immediate effects of beam trawling north east of Anglesey. They reported that, in stable sediments, the community significantly altered immediately after the trawl. The reduction in the abundance of Aphrodita aculeata and Nephtys spp. contributed significantly to the difference between trawled and control areas. However, no significant difference between trawled and control sites were detectable six months later, in part due to seasonal changes in the community during that period (Kaiser et al., 1998). In another experiment, only 7-8% of Aphrodita aculeata caught in a beam trawl died while the majority were not damaged (Kaiser & Spencer, 1995). Storms are also known to cause physical disturbance in sedimentary habitats. For example, moderate numbers of Aphrodita aculeata were reported to be stranded after the 1975 to 1976 storms in Red Wharf Bay, Anglesey (Rees et al., 1976). | Intermediate | High | Low | Low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceAphrodita aculeata is an active predator (Mettam, 1980) and probably capable of rapid burrowing. Therefore, if displaced to a suitable habitat, this species would probably burrow quickly to avoid predation. Therefore, an intolerance of low has been recorded. Recoverability is probably immediate. | Low | Immediate | Not sensitive | Low |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceResidues of organochlorines have been detected in the tissues of Aphrodita aculeata in the northwest of the Dutch coast, an area in which incineration occurred before 1991 (Dethlefsen et al., 1996). Residues of DDT and PCBs were detected however no information on their potential effects was noted. Little information on the effects of synthetic contaminants on Aphrodita aculeata was found. Bryan & Gibbs (1991) noted that polychaetes vary in their response, with some species exhibiting relative tolerance, while other species were moderately sensitive. However, in the absence of any further information, no assessment has been attempted. | No information | Not relevant | No information | Not relevant |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceBryan (1984) suggested that polychaetes are fairly resistant to heavy metals, based on the species studied. Short term toxicity in polychaetes was highest to Hg, Cu and Ag, declined with Al, Cr, Zn and Pb whereas Cd, Ni, Co and Se the least toxic. However, no information concerning Aphrodita aculeata was found and no assessment has been made. | No information | Not relevant | No information | Not relevant |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceSuchanek (1993) reviewed the effects of oil spills on marine invertebrates and concluded that, in general, on soft sediment habitats, infaunal polychaetes, bivalves and amphipods were particularly affected. Aphrodita aculeata inhabits sublittoral sediments which are unlikely to be affected by oil spills directly, although hydrocarbons adsorbed onto particulates may be deposited. However, no information concerning the effects of hydrocarbon contamination on Aphrodita aculeata was found and no assessment has been made. | No information | Not relevant | No information | Not relevant |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceNo information found | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceIncreasing nutrient levels may result in a change from the typical sediment community, to a community dominated by opportunist species (e.g. capitellids) with increased abundance but reduced species richness and in extreme situations to anoxic abiotic sediments (Pearson & Rosenberg, 1978). However, Aphrodita aculeata is a predator and unlikely to be directly affected, although it may suffer as a result of reduced oxygen levels in the sediment surface (see oxygenation below). However, no information concerning the effect of nutrient enrichment on Aphrodita was found and no assessment has been made. | No information | Not relevant | No information | Not relevant |
Increase in salinity [Show more]Increase in salinity
EvidenceKinne (1971b) suggested that marine polychaetes had limited capacity for osmoregulation and noted that the ionic concentration of the coelomic fluid in Aphrodite aculeata was very similar to that of ambient seawater. This evidence, although limited, suggests that it would be sensitive to an increase in salinity from full salinity, especially short term acute change. Therefore, an intolerance of high has been recorded, albeit at very low confidence. | High | High | Moderate | Very low |
Decrease in salinity [Show more]Decrease in salinity
EvidenceAphrodita aculeata has been recorded only from habitats with full salinity (JNCC, 1999). Kinne (1971b) suggested that marine polychaetes had limited capacity for osmoregulation and noted that the ionic concentration of the coelomic fluid in Aphrodite aculeata was very similar to that of ambient seawater. This evidence, although limited, suggests that it would be sensitive to a decrease in salinity, especially short term acute change. Therefore, an intolerance of high has been recorded, albeit at very low confidence. | High | High | Moderate | |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceAphrodita aculeata is a shallow burrower living in the surface sediment layer, which may be expected to be well oxygenated. However, no information on its tolerance of hypoxic conditions was found and no assessment has been made. | No information | Not relevant | No information | Not relevant |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceWhile the spines and scales are known to harbour several species of entoproct (see general biology), they are thought to be commensal rather than parasitic. No information concerning diseases in Aphrodita was found. | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceNo information on competing or potentially competing non-native species was found. | No information | Not relevant | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceAphrodita aculeata is unlikely to be the subject of targeted fisheries. However, it can be adversely affected by fishing activities for other species (see below) | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceLarge Aphrodita aculeata (> 7 cm in length) were reported to suffer up to 31% mortality due to beam or otter trawls in sandy sediments of the North Sea (Bergman & van Santbrink, 2000). No mortality was reported in smaller individuals, presumably because they could pass through the trawls unharmed. Bergman & van Santbrink (2000) also reported an annual mortality for Aphrodita aculeata of up to 20% in the Dutch sector of the North Sea in 1994. Kaiser et al. (1998) examined the immediate effects of beam trawling north east of Anglesey. They reported that, in stable sediments, the community significantly altered immediately after the trawl. The reduction in the abundance of Aphrodita aculeata and Nephtys spp. contributed significantly to the difference between trawled and control areas. However, no significant difference between trawled and control sites were detectable six months later, in part due to seasonal changes during that period (Kaiser et al., 1998). In another experiment, only 7-8% of Aphrodita aculeata caught in a beam trawl died while the majority were not damaged (Kaiser & Spencer, 1995). Veale et al. (2000) examined epifaunal assemblages in the North Irish Sea in areas subject to different intensities of scallop dredging. Areas subject to high fishing effort were correlated with decreased species richness and diversity. In addition, Aphrodita aculeata was not found in areas subject to high fishing effort but present in relatively high abundance in areas subject to medium or low fishing effort (Veale et al., 2000; Figure 3). | Intermediate | High | Low | Moderate |
Additional information
Recoverability. No information on larval development, recruitment or population dynamics in this species was found. Thorson's observations (Thorson, 1946) suggest that larvae either have a very short pelagic phase or no pelagic phase, perhaps developing on the sea bed. In Polynoe, eggs are incubated under the elytra, however, brooding has not been observed in Aphrodita (Fordham, 1925). Therefore, dispersal potential by larval transport is probably low. However, the adults are probably highly mobile, are common and widespread. Recruitment probably occurs by adult recolonization and subsequent good local recruitment from short-lived or benthic larvae. Therefore, recoverability may be high, although no evidence was found.
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
The iridescent lateral spines of Aphrodita have been shown to function as photonic crystals that strongly reflect different wavelengths of light depending on its angle of incidence (Parker et al., 2001). The study of its spines may provide clues to the design of photonic crystal fibres for use in communication (see Parker et al., 2001).Bibliography
Barnich, R. & Fiege, D., 2000. Review of the north east Atlantic and Mediterranean species of Aphrodita Linnaeus, 1758 and Aphroditella Roule, 1898 (Polychaeta: Aphroditidae). Ophelia, 53, 131-140.
Bryan, G.W. & Gibbs, P.E., 1991. Impact of low concentrations of tributyltin (TBT) on marine organisms: a review. In: Metal ecotoxicology: concepts and applications (ed. M.C. Newman & A.W. McIntosh), pp. 323-361. Boston: Lewis Publishers Inc.
Chambers, S.J., & Muir, A.I., 1997. Polychaetes: British Chrysopetaloidea, Pisionoidea and Aphroditoidea. London: Academic Press.[Synopses of the British Fauna, no. 54.]
Dethlefsen, V., Soeffker, K., Buether, H & Damm, U., 1996. Organochlorine compounds in marine organisms from the international North Sea incineration area Archive of Fishery and Marine Research, 44, 215-242.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Fordham, M.G.C., 1925. Aphrodite aculeta Liverpool: Liverpool Marine Biology Committee. [L.M.B.C. Memoirs XXVII].
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Kinne, O., 1971b. Salinity - invertebrates. In Marine Ecology: A Comprehensive, Integrated Treatise on Life in Oceans and Coastal Waters. Vol. 1 Environmental Factors, Part 2, pp. 821-995. London: John Wiley & Sons.
MBA (Marine Biological Association), 1957. Plymouth Marine Fauna. Plymouth: Marine Biological Association of the United Kingdom.
Mettam, C., 1971. Functional design and the evolution of the polychaete Aphrodite aculeata. Journal of Zoology (London), 163, 489-514.
Mettam, C., 1980. On the feeding habits of Aphrodita aculeata and commensal polynoids. Journal of the Marine Biological Association of the United Kingdom, 60, 833-834.
Parker, A.R., McPhedran, R.C., McKenzie, D.R., Botten, L.C. & Nicorovici, N.-A.P., 2001. Aphrodite's iridescence. Nature, 409, 36-37.
Rouse, G.W. & Pleijel, F., 2001. Polychaetes. New York: Oxford University Press.
Schroeder, P.C. & Hermans, C.O., 1975. Annelids: Polychaeta. In Reproduction of Marine Invertebrates. Vol. III. Annelids and Echiurans (ed. A.C. Giese & J.S. Pearse), pp. 1-213. New York: Academic Press.
Schroeder, P.C., 1989. Annelia - Polychaeta. In Reproductive biology of invertebrates, vol. IV, part A. Fertilization, development, and parental care (ed. K.G. Adiyodi & Rita Adiyodi), pp. 383-442. Chichester: John Wiley & Sons.
Thorson, G., 1946. Reproduction and larval development of Danish marine bottom invertebrates, with special reference to the planktonic larvae in the Sound (Øresund). Meddelelser fra Kommissionen for Danmarks Fiskeri- Og Havundersögelser, Serie: Plankton, 4, 1-523.
Veale, L.O., Hill, A.S., Hawkins, S.J. & Brand, A.R., 2000. Effects of long term physical disturbance by scallop fishing on subtidal epifaunal assemblages and habitats. Marine Biology, 137, 325-337.
Vella, G., Rushforth, I., Mason, E., Hough, A., England, R., Styles, P, Holt, T & Thorne, P., 2001. Assessment of the effects of noise and vibration from offshore windfarms on marine wildlife. Department of Trade and Industry (DTI) contract report, ETSU W/13/00566/REP. Liverpool: University of Liverpool., Department of Trade and Industry (DTI) contract report, ETSU W/13/00566/REP. Liverpool: University of Liverpool.
Datasets
Centre for Environmental Data and Recording, 2018. IBIS Project Data. Occurrence dataset: https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2016. Occurrence dataset: https://doi.org/10.15468/146yiz accessed via GBIF.org on 2018-09-27.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Lancashire Environment Record Network, 2018. LERN Records. Occurrence dataset: https://doi.org/10.15468/esxc9a accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-07-30
South East Wales Biodiversity Records Centre, 2018. SEWBReC Worms (South East Wales). Occurrence dataset: https://doi.org/10.15468/5vh0w8 accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 08/06/2007