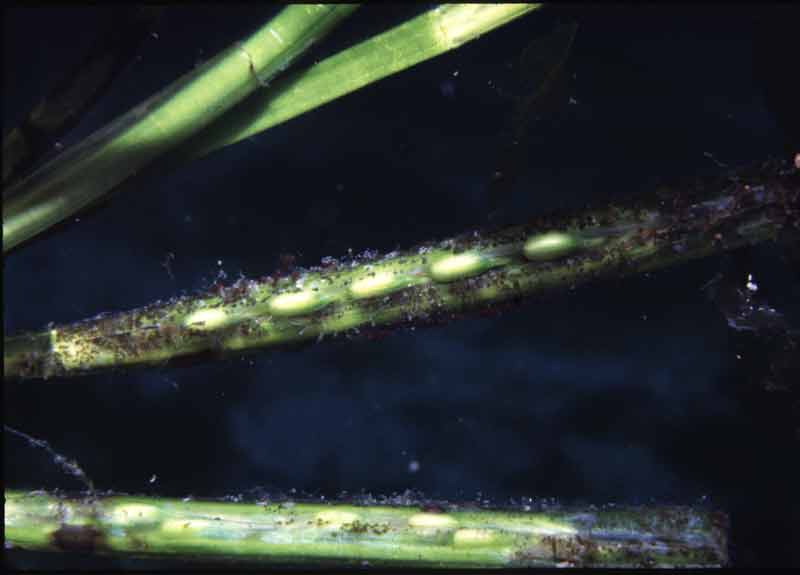
Common eelgrass (Zostera marina)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Dr Harvey Tyler-Walters | Refereed by | Dr Leigh Jones |
| Authority | Linnaeus, 1753 | ||
| Other common names | - | Synonyms | Zostera marina |
Summary
Description
Grass like flowering plant with dark green, long, narrow, ribbon shaped leaves 20-50 cm in length (exceptionally up to 2 m long) with rounded tips. Leaves shoot from a creeping rhizome that binds the sediment. Leaves and rhizomes contain air spaces, lacunae, that aid buoyancy. Numerous flowers occur on a reproductive shoot similar to those of terrestrial grasses. Forms dense swards in the subtidal, supports a diverse fauna and flora and may act as a nursery for fish and shellfish.
Recorded distribution in Britain and Ireland
Zostera marina has a wide but patchy distribution in southwest of England, the Solent and Isle of Wight on the south coast, Wales, western Ireland, western and eastern Scotland including Orkney and the Shetland Islands.
Global distribution
Widespread through the Atlantic and Pacific. It is the only seagrass species that extends into the Arctic Circle. It has a restricted distribution in the Mediterranean.
Habitat
Dense swards found primarily on sand to fine gravel in the subtidal, typically down to 4 m, in sheltered waters such as shallow inlets, bays, estuaries and saline lagoons.
Depth range
0 to 5 mIdentifying features
- Relatively thin, flattened, blade-like leaves, dark green in colour.
- Leaves usually 20-50 cm but up to 2 m in length, 4-10 mm wide, with 5-11 veins and rounded leaf tips, sometimes with a sharp point (mucronate).
- Leaf sheath forms a tube around stem.
- Reproductive shoot, terminal, branched and up to 15 m long.
- Seeds ovoid or ellipsoid with 16-25 distinct ribs.
- Rhizome with fibre bundles in the outermost layer of cortex.
Additional information
Other common names include, wigeon grass, broad leaved grass wrack, marlee, sedge and slitch. Perennial populations show a seasonal changes in leaf growth, the long leaves found in summer are replaced by shorter, slow growing leaves in winter. The morphological characteristics, especially leaf width may vary with environmental conditions (Phillips & Menez, 1988). In the UK literature Zostera marina is distinguished from Zostera angustifolia on the basis of morphology. However, outside the UK most authors consider Zostera angustifolia to be a phenotypic variant of Zostera marina. To avoid confusion only data relating to Zostera marina is presented.
Listed by
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Tracheophyta | Vascular plants (seagrasses, pondweeds, and reeds) |
| Class | Magnoliopsida | |
| Order | Alismatales | |
| Family | Zosteraceae | |
| Genus | Zostera | Eelgrasses |
| Authority | Linnaeus, 1753 | |
| Recent Synonyms | Zostera marina | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | |||
| Male size at maturity | |||
| Female size range | Medium-large(21-50cm) | ||
| Female size at maturity | |||
| Growth form | Foliose | ||
| Growth rate | 5m/year | ||
| Body flexibility | |||
| Mobility | Sessile, permanent attachment | ||
| Characteristic feeding method | Autotroph | ||
| Diet/food source | Photoautotroph | ||
| Typically feeds on | Not relevant | ||
| Sociability | Not relevant | ||
| Environmental position | Epifloral | ||
| Dependency | No text entered. | ||
| Supports | Substratum Entocladia perforans, a green alga; Rhodophysema georgii, a crustose red alga; and brown algae Halothrix lumbricalis, Leblondiella densa, Myrionema magnusii, Cladosiphon zosterae, and Punctaria crispata. | ||
| Is the species harmful? | No information | ||
Biology information
The stated growth rate refers to vegetative growth recorded in perennial populations whereas annual populations may expand at 30m / year in good conditions (Holt et al. 1997). The following species have been recorded only from seagrass leaves:
- the hydroid Laomedea angulata;
- the algae Rhodophysema georgii, Halothrix lumbricalis,Leblondiella densa, Myrionema magnusii, Cladosiphon zosterae, Punctaria crispata; and
- Cladosiphon contortus, which is larger and found primarily on Zosterasp.
- rhizomes.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Enclosed coast or Embayment, Estuary, Isolated saline water (Lagoon) |
| Biological zone preferences | Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Gravel / shingle, Muddy gravel, Muddy sand, Sandy mud |
| Tidal strength preferences | Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Sheltered, Very sheltered |
| Salinity preferences | Variable (18-40 psu) |
| Depth range | 0 to 5 m |
| Other preferences | |
| Migration Pattern | Non-migratory or resident |
Habitat Information
In 1920s and 1930s the previously extensive beds of eelgrass were severely reduced by an outbreak of 'wasting disease', which appears to affect sublittoral Zostera marina primarily. To date, recovery has been poor or slow. An exceptional bed of Zostera marina occurs in the clear waters of Ventry Bay, south-west Ireland and extends from 0.5 to 10m in depth and up to 13m deep in some patches. It should be noted that the global distribution of Zostera marina includes records of Zostera angustifolia which is considered synonymous outside the UK. It extends from Arctic Circle in northern Russia to near Gibraltar, Spain along the European coast. In has a restricted distribution in the Mediterranean, limited to northern parts of Adriatic and Aegean Seas, brackish etangs and lagoons in southern France. On the western Atlantic coast it extends from west coast of Alaska to North Carolina. In the Pacific it is recorded from Japan, Korea and from Alaska to Baja California, Mexico.
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | See additional information, Sexual, Vegetative |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | 100-1,000 |
| Generation time | 1-2 years |
| Age at maturity | 1-2 yr. |
| Season | May - September |
| Life span | 20-100 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Oviparous |
| Duration of larval stage | Not relevant |
| Larval dispersal potential | 100 -1000 m |
| Larval settlement period |
Life history information
Zostera sp. are perennials but may act as annuals under stressful conditions (Phillips & Menez 1988). Eelgrass reproduces sexually and vegetatively, i.e. by the growth of rhizome. Vegetative reproduction probably exceeds seedling recruitment except in areas of sediment disturbance (Reusch et al. 1998; Phillips & Menez 1988). Examination of the population structure of a Zostera marina bed in the Baltic Sea suggested that individual genotypes (vegetatively produced clones) may be up to 50 years old and further suggested that the eelgrass bed at that site had been present for at least 67 years (Reusch et al. 1998). Methods of dispersal include the following.
- All parts of the plant may float if they become detached from the substratum. Pieces of rhizome or shoots (if displaced by for example storm action) may take root if they settle on a suitable substratum.
- The generative stalk may be released together with the seed complement and may be carried great distances (Phillips & Menez, 1988).
- In New York, USA, Churchill et al. (1985) recorded 5-13% of seeds with attached gas bubbles and achieved an average dispersal distance of 21m and up to 200m in a few cases.
- Wildfowl may disperse seeds on their feet, or in their gut. For example, 30% of freshwater eelgrass (Naja marina) seeds fed to ducks in Japan survived and successfully germinated after passage through their alimentary canals and potentially transported 100-200km (Fishman & Orth 1996).
Phillips & Menez (1988) state that seedling mortality is extremely high. Fishman & Orth (1996) report that 96% of seeds were lost from uncaged test areas due to transport (dispersal) or predation. Ecological genetics studies of Zostera marina in False and Padilla Bays on the Pacific coast of the USA (Ruckelhaus 1998), detected genetic differentiation between intertidal and subtidal zones and between the bays. Estimates of gene flow suggested that seed dispersal was more important than pollen dispersal, effective migration (2.9 migrants/generation) occurred between the bays (14 km apart) and that the population subdivision was in part explained by disturbance and recolonization. Phillips & Menez (1988) note that seedlings rarely occur within the eelgrass bed except in areas cleared by storms, blow-out or excessive herbivory.
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceThe rhizome occupies the top 20cm of the substratum. Substratum loss will result in the loss of the shoots, rhizome and probably the seed bank. Recoverability will depend on recruitment from other populations. Although Zostera marina seed dispersal may occur over large distances, high seedling mortality and seed predation may significantly reduce effective recruitment. The slow recovery of Zostera populations since the 1920s - 30s outbreak of wasting disease suggests that, once lost, eelgrass beds take considerable time to re-establish. | High | Very low / none | Very High | Moderate |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceSediment disturbance, siltation, erosion and turbidity resulting from coastal engineering and dredging activities have been implicated in the decline of seagrass beds world wide ( Davison & Hughes 1998; Holt et al. 1997). Seagrasses are intolerant of smothering and typically bend over with addition of sediment and are buried in a few centimetres of sediment (Fonseca 1992). Recoverability will depend on recruitment from other populations. Although Zostera marina seed dispersal may occur over large distances, high seedling mortality and seed predation may significantly reduce effective recruitment. The slow recovery of Zostera populations since the 1920s - 30s outbreak of wasting disease suggests that, once lost, eelgrass beds take considerable time to re-establish. | High | Very low / none | Very High | Moderate |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceIncreased sediment erosion or accretion have been associated with loss of seagrass beds in the Australia, the Mediterranean and USA. Increased sediment availability may result in raised eelgrass beds, more likely to be exposed to low tide, desiccation and high temperatures. Seagrass beds demonstrate a balance of sediment accretion and erosion. Sediment deposited during summer months may be lost again due to winter storms, resuspension by grazing wildfowl, and increased erosion due to die back of leaves and shoots in autumn and winter. Seagrass beds should be considered intolerant of any activity that changes the sediment regime where the change is greater than expected due to natural events. | Intermediate | Moderate | Moderate | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidenceZostera marina is mainly subtidal and intolerant of desiccation compared to other species of eelgrass. If exposed at low tide the shoot bases are stiff and upright for a few centimetres, and leaf bases will be killed by 30 min exposure on a warm, sunny day (Holt et al. 1997). Even short periods of drying kills the flowers. However, if the rhizomes are undamaged the leaves will grow back but repeated exposure to desiccation may exhaust the energy stores in the rhizomes. Zostera marina may be more intolerant of activities that cause the sediment to drain or dry. | Intermediate | High | Low | Moderate |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceZostera marina that extend into the intertidal are likely to be highly intolerant of an increase in the emergence time (see desiccation). | Intermediate | High | Low | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceSeagrasses require sheltered environments, with gentle longshore currents and tidal flux. Where populations are found in moderately strong currents they are smaller, patchy and vulnerable to storm damage and blow outs. Increased water flow may also increase sediment erosion (see siltation above). Populations present in moderately strong currents may benefit from decreased water flow rates. | Intermediate | Moderate | Moderate | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidencePopulations of Zostera marina occur from the Mediterranean to Arctic Circle and are regarded as tolerant between about 5 - 30 deg C and tolerant of up to 20 deg C without stress. Therefore, they may tolerate the range of temperatures likely in the British Isles (Davison & Hughes 1998). However, intertidal populations may be damaged by frost (Hartog 1987). Populations at the edge of the range are likely to be more intolerant of temperature change. Phillips & Menez (1988) report death of seagrass as the result of a thermal plume in Biscayn Bay, Florida that raised ambient temperature by 5 degrees C, however, the species concerned were not cited. Long term temperature increase may increase the relative contribution of sexual reproduction and seed germination to population structure. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceLight attenuation limits the depth to which Zostera marina can grow and is a requirement for photosynthesis. Turbidity resulting from dredging and eutrophication caused a massive decline of Zostera populations in the Wadden Sea (Geisen et al. 1990). Seagrass populations are likely to survive increased turbidity for a month however prolonged increase in light attenuation will probably result in loss or damage of the population. | High | Very low / none | Very High | Moderate |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceSeagrasses require sheltered environments, with gentle longshore currents and tidal flux. Where populations are found in moderately strong currents they are smaller, patchy and vulnerable to storm damage and blow outs. Increased wave exposure may also increase sediment erosion (see siltation above). Populations present in moderately strong currents may benefit from decreased water flow rates. Small patchy populations or recently established population and seedling may be highly intolerant of increased wave action since they lack an extensive rhizome system. | High | Very low / none | Very High | Low |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidenceThe effect of sound waves and vibration on plants is poorly studied. However, it is likely that sound waves will have little effect at the benchmark levels suggested. | Tolerant | Not relevant | Not sensitive | High |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceContinuous shading will affect photosynthesis and therefore viability. However, occasional shading caused by surface movements of vessels at the level of this benchmark is unlikely to have an effect on seagrass beds. | Tolerant | Not relevant | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceSmall scale sediment disturbance may stimulate growth and small patches of sediment allow recolonization by seedlings (Davison & Hughes, 1998). However, seagrasses are not physically robust and rhizomes are likely to be damaged, and seeds buried too deep to germinate, by activities such as trampling, anchoring, digging, dredging, power boat and jet-ski wash (Fonseca, 1992). Suction dredging for cockles in Solway Firth removed Zostera in affected areas while Zostera was abundant in un-dredged areas (Perkins, 1988). Physical disturbance and removal of plants can lead to increased patchiness and destabilization of the seagrass bed, which in turn can lead to reduced sedimentation within the seagrass bed, increased erosion, and loss of larger areas of Zostera(Davison & Hughes, 1998). Therefore, the impact from a scallop dredge is likely to remove a proportion of the population and result in increased erosion of the bed. Therefore, intolerance has been recorded as intermediate. | Intermediate | Moderate | Moderate | Moderate |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceSeagrass rhizomes are easily damaged by trampling, anchoring, dredging and other activities that disturb the sediment. The seagrass bed is unlikely to survive displacement. However, Phillips & Menez (1988) reported that rhizomes and shoots can root and re-establish themselves if they settle on sediment long enough. | High | Low | High | Low |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceZostera marina is known to accumulate TBT but no damage was observable in the field (Williams et al., 1994). Naphthalene, pentachlorophenol, Aldicarb and Kepone reduce nitrogen fixation and may affect Zostera marina viability. Triazine herbicides (e.g. Irgarol) inhibit photosynthesis and sublethal effects have been detected. Terrestrial herbicides may damage eelgrass beds in the marine environment. For example the herbicide Atrazine is reported to cause growth inhibition and 50 percent mortality in Zostera marina exposed to 100 ppb (ng/ l) Atrazine for 21 days (Davison & Hughes 1998). | Intermediate | Moderate | Moderate | High |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceThe concentration and toxicity of heavy metals in salt marsh plants, including Zostera marina was reviewed by Williams et al. 1994. Growth of Zostera marina is inhibited by 0.32 mg/l Cu and 10 mg/l Hg but Cd, Zn, Cr and Pb had measurable but less toxic effects (Williams et al., (1994). Davison & Hughes (1998) report that Hg, Ni and Pb reduce nitrogen fixation which may affect viability. However, leaves and rhizomes accumulate heavy metals, especially in winter. Williams et al. (1994) did not observe any damage to Zostera marina in the field. | Low | Very high | Very Low | Moderate |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidence
| Low | Very high | Very Low | Moderate |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficient | No information | No information | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceWhere nutrients are limiting, additional low levels of nutrients may improve the growth of Zostera marina. The reported effects of nutrient enrichment include:
| High | Very low / none | Very High | Moderate |
Increase in salinity [Show more]Increase in salinity
EvidenceZostera sp. have a wide tolerance of salinity from 10 to 39 ppt (Davison & Hughes 1998). Germination in Zostera marina occurs over a range of salinities. | Low | Very high | Very Low | Low |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceThe effects of oxygen concentration on the growth and survivability of Zostera marina are not reported in the literature. Zostera marina leaves contain air spaces (lacunae) and oxygen is transported to the roots where it permeates into the sediment, resulting in a oxygenated microzone. This enhances the uptake of nitrogen. The presence of air spaces suggests that seagrass may be tolerant of low oxygen levels in the short term, however, prolonged deoxygenation, especially if combined with low light penetration and hence reduced photosynthesis may have an effect. | Low | Very high | Very Low | Low |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceA major outbreak of wasting disease resulted in significant declines of Zostera marina beds in 1920s to 1930s. Wasting disease is thought to be caused by the marine fungus, Labyrinthula macrocystis. The disease is less likely at low salinities however, Zostera marina prefers full salinities. The disease causes death of leaves and after 2-3 seasons death of regenerative shoots, rhizomes and loss of up to 90 percent of the population. | High | Very low / none | Very High | High |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceSpartina anglica (a cord grass) is an invasive pioneer species, a hybrid of introduced and native cord grass species. Its rapid growth consolidates sediment, raises mudflats and reduces sediment availability elsewhere. It has been implicated in the reduction of common eelgrass cover in Lindisfarne, Northumberland due to encroachment and changes in sediment dynamics. Wire weed (Sargassum muticum) invades open substratum and may prevent recolonization of areas of eelgrass beds left open by disturbance (Davison & Hughes 1998). Zostera marina and Sargassum muticum may compete for space in the lower shore lagoons of the Solent. However, evidence for competition is conflicting and requires further research. If the invasive species prevent recolonization then recoverability from other factors will be reduced. | Intermediate | Low | High | Moderate |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceWildfowl grazing can consume significant amounts of seagrass and reduce cover mainly in autumn and winter. Grazing is probably part of the natural seasonal fluctuation in seagrass cover and Zostera sp. can recover from normal grazing. However, where a bed is stress by other factors it may not be able to withstand grazing (Holt et al. 1997; Davison & Hughes 1998). Eelgrass rhizomes are easily damaged by trampling, anchoring, dredging and other activities that disturb the sediment. The seagrass bed is unlikely to survive displacement or extraction. However, Phillips & Menez (1988) reported that rhizomes and shoots can root and re-establish themselves if they settle on sediment long enough. | Intermediate | Moderate | Moderate | Low |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceSeagrass rhizomes are easily damaged by trampling, anchoring, dredging and other activities that disturb the sediment. Seeds may be buried too deep to germinate. Mechanical dredging of cockles in Solway Firth, in intertidal Zostera beds, resulted in the loss of the seagrass bed and was closed. Dredging for bivalves has been implicated in the decline of seagrass beds in the Dutch, Wadden Sea. Damage after the Sea Empress oil spill was reported as limited to the ruts left by clean up vehicles. | Intermediate | Moderate | Moderate | Moderate |
Additional information
Importance review
Policy/legislation
| Designation | Support |
|---|---|
| Berne Convention | Appendix I |
| IUCN Red List | Least Concern (LC) |
Status
| National (GB) importance | Not rare or scarce | Global red list (IUCN) category | Least Concern (LC) |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
In the Mediterranean, Zostera marina is strictly protected under the Berne Convention. In the UK, it did not have a species Biodiversity Action Plan (BAP) but was covered by a Habitat Action Plan (HAP). Zostera is an important component of the diet of Brent geese (Branta bernicla), wigeon (Anas penelope), mute and whooper swans (Cygnus olor and Cygnus cygnus). The Brent goose population in Europe declined as a result of the decline in eelgrass populations due to wasting disease. Zostera noltii has replaced Zostera marina as the preferred food species. Seagrasses have been put to a number of uses in the past, for example, sound proofing, insulation, roofing thatch, binding soil, packaging, basket weaving and in the manufacture of 'coir' matting (see Kuelan, 1999 for review).
Bibliography
Anonymous, 1999p. Seagrass beds. Habitat Action Plan. In UK Biodiversity Group. Tranche 2 Action Plans. English Nature for the UK Biodiversity Group, Peterborough., English Nature for the UK Biodiversity Group, Peterborough.
Burkholder, J.M., Mason, K.M. & Glasgow, H.B. Jr., 1992. Water-column nitrate enrichment promotes decline of eelgrass Zostera marina: evidence from seasonal mesocosm experiments. Marine Ecology Progress Series, 81, 163-178.
Churchill, A.C., Nieves, G. & Brenowitz, A.H., 1985. Floatation and dispersal of eelgrass seeds by gas bubbles. Estuaries, 8, 352-354.
Davison, D.M. & Hughes, D.J., 1998. Zostera biotopes: An overview of dynamics and sensitivity characteristics for conservation management of marine SACs, Vol. 1. Scottish Association for Marine Science, (UK Marine SACs Project)., Scottish Association for Marine Science, (UK Marine SACs Project),Vol. 1. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/zostera.pdf
Den Hartog, C., 1970. The sea-grasses of the world. Amsterdam: North Holland Publishing Company.
Den Hartog, C., 1987. "Wasting disease" another dynamic phenomena in Zostera beds. Aquatic Botany, 27, 3 -14.
Den Hartog, C., 1994. Suffocation of a littoral Zostera bed by Enteromorpha radiata. Aquatic Botany, 47, 21-28.
Fishman, J.R. & Orth, R.J., 1996. Effects of predation on Zostera marina L. seed abundance. Journal of Experimental Marine Biology and Ecology, 198, 11-26.
Fonseca, M.S., 1992. Restoring seagrass systems in the United States. In Restoring the Nation's Marine Environment (ed. G.W. Thayer), pp. 79 -110. Maryland: Maryland Sea Grant College.
Giesen, W.B.J.T., Katwijk van, M.M., Hartog den, C., 1990a. Eelgrass condition and turbidity in the Dutch Wadden Sea. Aquatic Botany, 37, 71-95. DOI https://doi.org/10.1016/0304-3770(90)90065-S
Guiry, M.D. & Nic Dhonncha, E., 2000. AlgaeBase. World Wide Web electronic publication http://www.algaebase.org, 2000-01-01
Holt, T.J., Hartnoll, R.G. & Hawkins, S.J., 1997. The sensitivity and vulnerability to man-induced change of selected communities: intertidal brown algal shrubs, Zostera beds and Sabellaria spinulosa reefs. English Nature, Peterborough, English Nature Research Report No. 234.
Jones, L.A., Hiscock, K. & Connor, D.W., 2000. Marine habitat reviews. A summary of ecological requirements and sensitivity characteristics for the conservation and management of marine SACs. Joint Nature Conservation Committee, Peterborough. (UK Marine SACs Project report.). Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/marine-habitats-review.pdf
Kuelan, van M., 1999. Human uses of seagrass. http://possum.murdoch.edu.au/~seagrass/seagrass_uses.html, 2000-01-01
Perkins, E.J., 1988. The impact of suction dredging upon the population of cockles Cerastoderma edule in Auchencairn Bay. Report to the Nature Conservancy Council, South-west Region, Scotland, no. NC 232 I).
Phillips, R.C., & Menez, E.G., 1988. Seagrasses. Smithsonian Contributions to the Marine Sciences, no. 34.
Reusch, T.B.H., Stam, W.T., & Olsen, J.C. 1998. Size and estimated age of genets in eelgrass, Zostera marina, assessed with microsatellite markers. Marine Biology, 133, 519-525.
Rucklehaus, M.H., 1998. Spatial scale of genetic structure and an indirect estimate of gene flow in eelgrass, Zostera marina. Evolution, 52, 330-343
Stewart, A., Pearman, D.A. & Preston, C.D., 1994. Scarce plants in Britain. Joint Nature Conservation Committee, Peterborough.
Williams, T. P., Bubb, J. M. & Lester, J. N., 1994. Metal accumulation within salt-marsh environments - a review. Marine Pollution Bulletin, 28 (5), 277-290. DOI https://doi.org/10.1016/0025-326x(94)90152-x
Datasets
Botanical Society of Britain & Ireland, 2018. Other BSBI Scottish data up to 2012. Occurrence dataset: https://doi.org/10.15468/2dohar accessed via GBIF.org on 2018-09-25.
Botanical Society of Britain & Ireland, 2018. Scottish SNH-funded BSBI records. Occurrence dataset: https://doi.org/10.15468/llasrt accessed via GBIF.org on 2018-09-25.
Botanical Society of Britain & Ireland, 2018. Welsh BSBI data (ex-VPDB dataset) at hectad resolution. Occurrence dataset: https://doi.org/10.15468/rsvnif accessed via GBIF.org on 2018-09-25.
Bristol Regional Environmental Records Centre, 2017. BRERC species records recorded over 15 years ago. Occurrence dataset: https://doi.org/10.15468/h1ln5p accessed via GBIF.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. Ulster Wildlife Snorkel Safaris. Occurrence dataset: https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Isle of Wight Local Records Centre, 2017. Isle of Wight Notable Species. Occurrence dataset: https://doi.org/10.15468/sm4ety accessed via GBIF.org on 2018-09-27.
Manx Biological Recording Partnership, 2017. Isle of Man wildlife records from 01/01/2000 to 13/02/2017. Occurrence dataset: https://doi.org/10.15468/mopwow accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2018. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset: https://doi.org/10.15468/aru16v accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2022. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset:https://doi.org/10.15468/aru16v accessed via GBIF.org on 2024-09-27.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
Norfolk Biodiversity Information Service, 2017. NBIS Records to December 2016. Occurrence dataset: https://doi.org/10.15468/jca5lo accessed via GBIF.org on 2018-10-01.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-02
Royal Botanic Garden Edinburgh, 2018. Royal Botanic Garden Edinburgh Herbarium (E). Occurrence dataset: https://doi.org/10.15468/ypoair accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. SEWBReC Vascular Plants (South East Wales). Occurrence dataset: https://doi.org/10.15468/7qjujd accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. Dr Mary Gillham Archive Project. Occurance dataset: http://www.sewbrec.org.uk/ accessed via NBNAtlas.org on 2018-10-02
Suffolk Biodiversity Information Service., 2017. Suffolk Biodiversity Information Service (SBIS) Dataset. Occurrence dataset: https://doi.org/10.15468/ab4vwo accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 02/08/2008