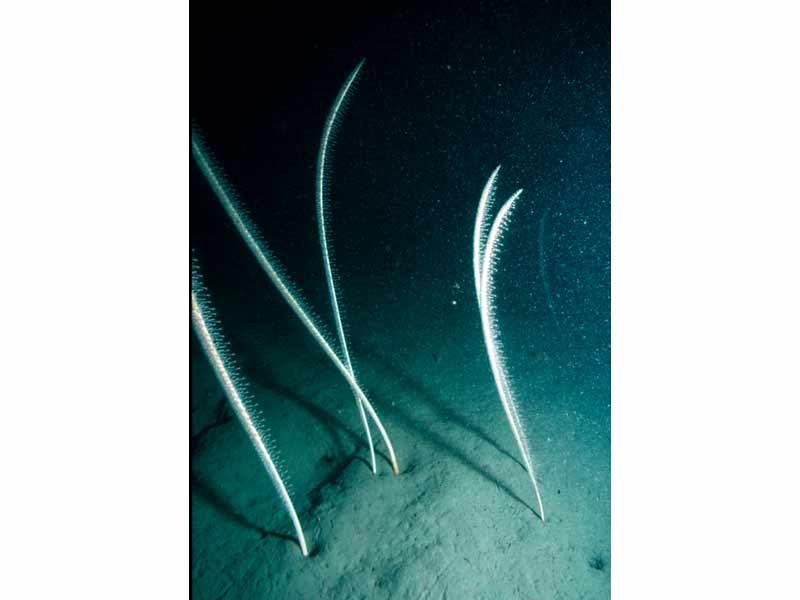
The tall sea pen (Funiculina quadrangularis)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Olwen Ager | Refereed by | This information is not refereed |
| Authority | (Pallas, 1766) | ||
| Other common names | - | Synonyms | - |
Summary
Description
A tall, narrow sea pen, which can exceed 2 metres in height. It has a calcareous white axis, square in section. The polyps are irregularly arranged along the axis or tend to form oblique rows. They are white or pale pink in colour.
Recorded distribution in Britain and Ireland
West and north coasts of Ireland and Scotland.Global distribution
Funiculina quadrangularis occurs in the North Atlantic and Mediterranean. It has been recorded in New Zealand (Manuel, 1988) and Japan (Fujita & Ohta, 1988).Habitat
Found in muddy substrata on sheltered coasts, especially in sea lochs. Sublittoral to deep offshore water.Depth range
20-2000mIdentifying features
- Axis white, diagnostically box like in section.
- Lower quarter of stem forms a smooth peduncle, upper one third is curved.
- Autozooids irregularly arranged on rachis or in short oblique rows, retractile within toothed calyces.
- Autozooids often pink.
Additional information
-none-Listed by
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Cnidaria | Sea anemones, corals, sea firs & jellyfish |
| Class | Anthozoa | Sea anemones, soft & cup corals, sea pens & sea pansies |
| Order | Scleralcyonacea | |
| Family | Funiculinidae | |
| Genus | Funiculina | |
| Authority | (Pallas, 1766) | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Moderate density | ||
| Male size range | 1.5-2.1m | ||
| Male size at maturity | |||
| Female size range | Large(>50cm) | ||
| Female size at maturity | |||
| Growth form | Pinnate | ||
| Growth rate | |||
| Body flexibility | Low (10-45 degrees) | ||
| Mobility | |||
| Characteristic feeding method | Passive suspension feeder | ||
| Diet/food source | Detritivore, Planktotroph | ||
| Typically feeds on | Plankton and organic particles | ||
| Sociability | Solitary | ||
| Environmental position | Epibenthic | ||
| Dependency | No information found. | ||
| Supports | Substratum the brittlestar Asteronyx loveni and the isopod crustacean Astacilla longicornis . | ||
| Is the species harmful? | No information | ||
Biology information
Flexibility. Eno et al. (1996) found that the tall sea pen bends away from lobster creels dropped on them in a passive response to the pressure wave travelling ahead of the pot. However, the axial rod of this sea pen is brittle, making the species vulnerable to physical disturbance (Greathead et al., 2007).
Associated species. The deep-water brittlestar, Asteronyx loveni, which has been recorded sporadically from the west coast of Scotland (Hughes, 1998b), is known to use its arms to cling to Funiculina quadrangularis (Fujita & Ohta, 1988).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast, Sea loch or Sea lough |
| Biological zone preferences | Lower circalittoral, Upper circalittoral |
| Substratum / habitat preferences | Mud, Muddy sand |
| Tidal strength preferences | Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Extremely sheltered, Sheltered, Ultra sheltered, Very sheltered |
| Salinity preferences | Full (30-40 psu) |
| Depth range | 20-2000m |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
Although previously recorded only within sea lochs, a recent study by Greathead et al. (2007) found the species distribution to include the outer mouths of sea lochs, and areas of open water such as North and South Minch, and the Cuillin Sound where water depth exceeds 100 m.This sea pen has been recorded in high abundance in Loch Sunart, Loch Teacuis, Loch Duich and Loch a'Chairn Bhain from the mainland and in Loch Seaforth on Lewis.
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | No information |
| Fecundity (number of eggs) | No information |
| Generation time | See additional information |
| Age at maturity | see additional information |
| Season | Insufficient information |
| Life span | See additional information |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | See additional information |
| Duration of larval stage | No information |
| Larval dispersal potential | No information |
| Larval settlement period | Insufficient information |
Life history information
- Sexes in sea pens are separate; each colony of polyps are either male or female.
- Hughes (1998a) suggests, using the information from other species of seapen, that Funiculina quadrangularis may follow a similar pattern of patchy recruitment, slow growth and a long lifespan. Isolated populations are likely to be self-seeding, and hence susceptible to local extinction if their environments are disrupted (Hughes, 1998).
- Birkeland (1974) found the lifespan of Ptilosarcus gurneyi to be 15 years, reaching sexual maturity between the ages of 5 and 6 this may be similar in Funiculina quadrangularis.
It was estimated by Wilson et al. (2002), that larger specimens of a tall sea pen (Halipteris willemoesi) in the Bering Sea were 44 years old, with a growth rate of 3.6 - 6.1 cm per year. - The developmental mechanism for Funiculina quadrangularis is unknown, however large size of larvae in many sea pens is indicative of lecithotrophic larva. Sea pen fecundity is high, and varies between 30,000-200,000 ocytes per colony (Edwards & Moore, 2008)
Another British sea pen, Pennatula phosphorea, spawns in summer (July-August), with each polyp releasing approximately 50 ocytes, having a fecundity of 40,000 ocytes per colony for medium to large specimens (Edwards & Moore, 2008).
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceAs Funiculina quadrangularis lives in the substratum, substratum loss will lead to loss of the population. Therefore, intolerance has been recorded as high. | High | Low | High | Moderate |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceSmothering would be expected to be caused by high levels of siltation. Funiculina quadrangularis is very tall and would protrude above the sediment, therefore an intolerance of low as been recorded. Recoverability would be expected to be immediate, therefore the species is assessed as not sensitive. | Low | Immediate | Not sensitive | Moderate |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceThe effect of an increased deposit of fine silt is unclear but it is possible that feeding structures may become clogged (Jones et al., 2000). Kinnear et al. (1996) found that mud particles adhering to Funiculina quadrangularis were quickly removed due to large amounts of mucus produced. Also, an increase in suspended sediment may provide an increase in food availiabity for Funiculina quadrangularis. Therefore an intolerance of low has been recorded. Recovery on return to normal conditions is likely to be immediate, therefore the species is assessed as not sensitive. | Low | Immediate | Not sensitive | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceThe effects of a decrease in suspended sediment levels are unclear. It is possible that this factor may reduce the amount of particulate matter available as food for Funiculina quadrangularis, which may affect viability but is unlikely to be fatal at the level of the benchmark. Therefore an intolerance of low has been recorded. Recovery on return to normal conditions is likely to be immediate, therefore the species is assessed as not sensitive. | Low | Immediate | No information | |
Desiccation [Show more]Desiccation
EvidenceFuniculina quadrangularis is a sub-tidal species and is therefore likely to be highly intolerant of desiccation. However, the species is found in the circalittoral zone (below 20 m) where desiccation is unlikely to occur, so not relevant has been recorded. | Not relevant | Not relevant | Not relevant | Not relevant |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceFuniculina quadrangularis is a sub-tidal species and is therefore likely to be highly intolerant of emergence. However, the species is found in the circalittoral zone (below 20 m) where any emergence is highly unlikely to occur, so not relevant has been recorded. | Not relevant | Not relevant | Not relevant | Not relevant |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceFuniculina quadrangularis is a sub-tidal species and is therefore likely to be highly intolerant of emergence. However, the species is found in the circalittoral zone (below 20 m) where any emergence is highly unlikely to occur, so not relevant has been recorded. | Not relevant | Not relevant | Not relevant | Not relevant |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceAreas where Funiculina quadrangularis is found tend to be physically sheltered with low or very low water flow rates. No information was found about effects of increased water flow rate on Funiculina quadrangularis. However, experiments on another sea pen (Virgularia mirabilis) showed that, as water flow increased, the polyps faced away from the current to face downstream. Increasing current led to the stalk bending over and the pinnae becoming pushed together, which may result in feeding inhibition. Eventually the sea pen withdrew into the sediment (Hiscock, 1983). Furthermore, Funiculina quadrangularis is large with a short peduncle and increased flow may drag the sea pen out of the sediment. If feeding was inhibited, or if the sea pen was displaced for an excessive amount of time, survival and distribution of Funiculina quadrangularis may be affected (Hiscock, 1983). Therefore intolerance has been recorded as high. | High | Low | High | Moderate |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceAreas where Funiculina quadrangularis is found tend to be physically sheltered with low or very low water flow rates, however, as this sea pen is a suspension feeder a decrease in water flow rate may effect feeding. Therefore an intolerance of intermediate has been recorded. | Intermediate | Moderate | Moderate | Moderate |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceNo information was found of the upper or lower thermal limits of Funiculina quadrangularis. Funiculina quadrangularis occurs in thermally stable conditions, with annual temperature variation between 5 - 15°C (Jones et al., 2001). This species is subtidal where wide variations in temperature are not common, so may be intolerant of short term changes in temperature. Therefore intolerance has been recorded as intermediate. | Intermediate | Moderate | Moderate | Low |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceNo information was found of the upper or lower thermal limits of Funiculina quadrangularis. Funiculina quadrangularis occurs in thermally stable conditions, with annual temperature variation between 5 - 15 (Jones et al., 2001). This species is subtidal where wide variations in temperature are not common, so may be intolerant of short term changes in temperature. Therefore intolerance has been recorded as intermediate. | Intermediate | Moderate | Moderate | Low |
Increase in turbidity [Show more]Increase in turbidity
EvidenceNo information could be found about the sensitivity of Funiculina quadrangularis to light. Another sea pen Virgularia mirabilis, was not found to be sensitive to light (Hoare & Wilson, 1977). | No information | No information | Not sensitive | No information |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceNo information could be found about the intolerance of Funiculina quadrangularis to light. Another sea pen Virgularia mirabilis was found to be insensitive to light (Hoare & Wilson, 1977). | No information | No information | Moderate | No information |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceFuniculina quadrangularis is found in areas with little or no wave exposure. Virgularia mirabilis was found to be smaller and less common where wave exposure increased (Hoare & Wilson, 1977). It is likely that an increase in exposure ranking of 2 or more would kill Funiculina quadrangularis. Therefore, an intolerance of high has been recorded. | High | Low | High | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceFuniculina quadrangularis is found in areas with little or no wave exposure so it is unlikely that a decrease in wave exposure would occur. Therefore, not relevant has been recorded. | Not relevant | Not relevant | Not relevant | Not relevant |
Noise [Show more]Noise
EvidenceFuniculina quadrangularis probably has a very limited if any ability for detection of noise vibrations, therefore tolerant has been recorded, and the species is assessed as not sensitive. | Tolerant | Not relevant | Not sensitive | Very low |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceFuniculina quadrangularis probably has a very limited if any ability for visual perception, and is not known to be sensitive to light (Greathead et al., 2007). It is unlikely the sea pen will be sensitive to visual presence. Therefore tolerant has been recorded, and the species is assessed as not sensitive. | Tolerant | Not relevant | Not sensitive | Very low |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceSea pens project above the surface of the seabed and so are likely to be vulnerable to physical disturbance (Eno et al., 2001). Eno et al. (1996) dropped creels onto sea pens. They were seen to bend away in response to the pressure wave travelling ahead of the dropping creel, therefore reducing the chance the top of the sea pen would be struck causing fracture of the colony. Kinnear et al. (1996) conducted creel dragging experiments. The majority of Funiculina quadrangularis that were displaced from the sediment recovered after 72 hours. However, fishing gear such as a scallop dredge (see benchmark) or, more likely for this species, a Nephrops trawl, is likely to have a more severe impact. Sea pens are likely to be particularly vulnerable to damage from trawls, and have been reported as numerous in bycatch from otter trawls in the Bering sea (Brodeur 2001; Wilson et al., 2002). It is possible that the apparent absence of Funiculina quadrangularis from many open coast Nephrops grounds may be a consequence of its susceptibility to trawl damage (Connor, pers. comm. in Hughes, 1998a). For example when the sea pen Halipteris willemoesi becomes entangled in prawn trapping gear, 50% of sea pen colonies are damaged (Troffe et al., 2005). | High | Moderate | Moderate | Moderate |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceFuniculina quadrangularis can not retract into the sediment so it is likely to be removed from the seabed by the action of fishing gear. Removal experiments by Kinnear et al. (1996) showed when a sea pen was displaced it could reburrow as long as its foot remains in contact with the mud. Therefore, intolerance has been recorded as low. Recovery would take less that 72 hrs, so recoverability is recorded as immediate, yielding a sensitivity value of not sensitive. | Low | Immediate | Not sensitive | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceInsufficient | No information | No information | No information | Not relevant |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceInsufficient | No information | No information | No information | Not relevant |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceInsufficient | No information | No information | No information | Not relevant |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceNo information was found for Funiculina quadrangularis in relation to intolerance to radionuclides. However another species, Virgularia mirabilis, occurred in high density (10/m) at a sampling station immediately offshore from the Sellafield outfall pipeline in the Irish Sea (Hughes & Atkinson, 1997). Bottom sediments in this area contains particles of long half life radionuclides from liquid effluent, so intolerance has been recorded as low. Recovery is likely to be high, therefore the species is assessed as low sensitivity. | Low | High | Low | Low |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceA low level of nutrient enrichment is likely to be beneficial to Funiculina quadrangularis. However, because the distribution of the species is limited to relatively small areas within semi-enclosed water bodies, organic encrichment is expected to have adverse effects (Hughes, 1998). Jones et al. (2000) found Funiculina quadrangularis absent from de-oxygenated areas which are characterised by nutrient enrichment resulting in a hypoxic bacterial community. Salmon farming has been identified as the greatest source of organic pollution in Scottish sea lochs (Hiscock et al., 2005). Therefore intolerance has been recorded as intermediate. Recovery is recorded as high, hence a low sensitivity value. | Intermediate | High | Low | Moderate |
Increase in salinity [Show more]Increase in salinity
EvidenceFuniculina quadrangularis is found in fully saline conditions and it is unlikely that it would be exposed to hypersaline conditions, therefore, not relevant has been recorded. | Not relevant | Not relevant | Not relevant | Not relevant |
Decrease in salinity [Show more]Decrease in salinity
EvidenceFuniculina quadrangularis is found only in fully saline conditions so it is likely that the sea pen would be intolerant of a decrease in salinity. Therefore an intolerance of high has been recorded. | High | Low | Not relevant | |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceFuniculina quadrangularis is found in sea lochs where stratification of the water column is likely to occur during warm temperatures so it may be able to tolerate some reduction in oxygenation. However, Jones et al. (2000) found the sea pen absent from de-oxygenated areas which are characterised by nutrient enrichment resulting in a hypoxic bacterial community. Therefore intolerance has been recorded as intermediate. | Intermediate | Moderate | Moderate | Low |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceNo information was found on diseases of Funiculina quadrangularis. | No information | No information | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceInsufficient | No information | No information | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceFuniculina quadrangularis is unlikely to be subject to extraction, therefore, this factor has been assessed as not relevant. This sea pen can not retract into the sea bed which may mean it can be extracted through dredging activity. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceTrawling for the Norway lobster (Nephrops norvegicus) in the open sea and more accessible sea lochs may have removed populations of Funiculina quadrangularis. Large quantities of Funiculina quadrangularis axis have been observed below Dunstaffnage pier, presumably discarded bycatch (Hiscock, pers comm.). Other species of sea pen have also been recorded as numerous bycatch in trawls from the Bering sea (Brodeur 2001; Wilson et al., 2002). It is likely that physical disturbance from demersal fishing activities poses the greatest threat to Funiculina quadrangularis, (Greathead et al., 2007), and due to the fragmented distribution and possibly limited larval dispersal, recovery if unlikely (Hughes, 1998a). | High | Low | High | Low |
Additional information
Recoverability. There have been no long-term studies of British sea pen populations so the assessment of intolerance and recoverability is based on the available information for other species of sea pen. However, Funiculina quadrangularis is the most sensitive of the British sea pens (Hughes, 1998; Greathead et al., 2007). There is very little information on the life cycles and population dynamics of British sea pens. From the limited information on other species, Hughes (1998) suggested a pattern of patchy recruitment, slow growth and a long life span. Recoverability would depend on recolonization from other populations. Larval settlement is likely to be limited and patchy in space and time, with possibly no recruitment for several consecutive years.
Importance review
Policy/legislation
| Designation | Support |
|---|---|
| UK Biodiversity Action Plan Priority | Yes |
| Species of principal importance (England) | Yes |
Status
| National (GB) importance | Not rare or scarce | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
-none-Bibliography
Birkeland, C., 1974. Interactions between a seapen and seven of its predators. Ecological Monographs, 44, 211-232. DOI https://doi.org/10.2307/1942312
Brodeur, R.D., 2001. Habitat-specific distribution of Pacific Ocean perch (Sebastes alutus) in Pribilof Canyon, Bering Sea. Continental Shelf Research, 21, 207-224.
Davies, J., 1990. Sublittoral survey of Loch Sunart and Loch Teacus Joint Nature Conservation Committee Report, no. 1075.
Edwards, C.B. & Moore, C.G., 2008. Reproduction in the sea pen Pennatula phosphorea (Anthozoa: Pennatulacea) from the west coast of Scotland Marine Biology 155:303–314
Eno, N.C., Clark, R.A. & Sanderson, W.G. (ed.) 1997. Non-native marine species in British waters: a review and directory. Peterborough: Joint Nature Conservation Committee.
Eno, N.C., MacDonald, D. & Amos, S.C., 1996. A study on the effects of fish (Crustacea/Molluscs) traps on benthic habitats and species. Final report to the European Commission. Study Contract, no. 94/076.
Eno, N.C., MacDonald, D.S., Kinnear, J.A.M., Amos, C.S., Chapman, C.J., Clark, R.A., Bunker, F.S.P.D. & Munro, C., 2001. Effects of crustacean traps on benthic fauna ICES Journal of Marine Science, 58, 11-20. DOI https://doi.org/10.1006/jmsc.2000.0984
Fujita, T. & Ohta, S., 1988. Photographic observations of the lifestyle of deep-sea ophiuroid Asteronynx loveni (Echinodermata). Deep Sea Research, 35, 2029-2043.
Greathead, C.F., Donnan, D.W., Mair, J.M. & Saunders, G.R., 2007. The sea pens Virgularia mirabilis, Pennatula phosphorea and Funiculina quadrangularis: distribution and conservation issues in Scottish waters. Journal of the Marine Biological Association, 87, 1095-1103. DOI https://doi.org/10.1017/S0025315407056238
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hiscock, K., 1983. Water movement. In Sublittoral ecology. The ecology of shallow sublittoral benthos (ed. R. Earll & D.G. Erwin), pp. 58-96. Oxford: Clarendon Press.
Hiscock, K., Sewell, J. & Oakley, J., 2005. Marine Health Check 2005. A report to guage the health of the UK’s sea-life. Godalming, WWF-UK.
Hoare, R. & Wilson, E.H., 1977. Observations on the behaviour and distribution of Virgularia mirabilis O.F. Müller (Coelenterata: Pennatulacea) in Holyhead harbour. In Proceedings of the Eleventh European Symposium on Marine Biology, University College, Galway, 5-11 October 1976. Biology of Benthic Organisms, (ed. B.F. Keegan, P.O. Ceidigh & P.J.S. Boaden, pp. 329-337. Oxford: Pergamon Press. Oxford: Pergamon Press.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Howson, C.M., Connor, D.W. & Holt, R.H.F., 1994. The Scottish sealochs - an account of surveys undertaken for the Marine Nature Conservation Review. Joint Nature Conservation Committee Report, No. 164 (Marine Nature Conservation Review Report MNCR/SR/27)., Joint Nature Conservation Committee Report, No. 164 (Marine Nature Conservation Review Report MNCR/SR/27).
Hughes, D.J. & Atkinson, R.J.A., 1997. A towed video survey of megafaunal bioturbation in the north-eastern Irish Sea. Journal of the Marine Biological Association of the United Kingdom, 77, 635-653.DOI https://doi.org/10.1017/s0025315400036122
Hughes, D.J., 1998a. Sea pens & burrowing megafauna (volume III). An overview of dynamics and sensitivity characteristics for conservation management of marine SACs. Natura 2000 report prepared for Scottish Association of Marine Science (SAMS) for the UK Marine SACs Project., Scottish Association for Marine Science. (UK Marine SACs Project). Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/seapens.pdf
Hughes, D.J., 1998b. Subtidal brittlestar beds. An overview of dynamics and sensitivity characteristics for conservation management of marine SACs. Natura 2000 report prepared for Scottish Association of Marine Science (SAMS) for the UK Marine SACs Project., Scottish Association for Marine Science. (UK Marine SACs Project, Vol. 3). Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/britstar.pdf
Jones, L.A., Hiscock, K. & Connor, D.W., 2000. Marine habitat reviews. A summary of ecological requirements and sensitivity characteristics for the conservation and management of marine SACs. Joint Nature Conservation Committee, Peterborough. (UK Marine SACs Project report.). Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/marine-habitats-review.pdf
Kinnear, J.A.M., Barkel, P.J., Mojseiwicz, W.R., Chapman, C.J., Holbrow, A.J., Barnes, C. & Greathead, C.F.F., 1996. Effects of Nephrops creels on the environment. Fisheries Research Services Report No. 2/96, 24 pp. Available from https://www2.gov.scot/Uploads/Documents/frsr296.pdf
Manuel, R.L., 1988. British Anthozoa. Synopses of the British Fauna (New Series) (ed. D.M. Kermack & R.S.K. Barnes). The Linnean Society of London [Synopses of the British Fauna No. 18.]. DOI https://doi.org/10.1002/iroh.19810660505
Troffe, P.M., Levings, C.D., Piercey, G.B.E. & Keong, V., 2005. Fishing gear effects and ecology of the sea whip Halipteris willemoesi (Cnidaria: Octocorallia: Pennatulacea)) in British Columbia, Canada: preliminary observations Aquatic Conservation: Marine and Freshwater Ecosystems, 15, 523-533. DOI https://doi.org/10.1002/aqc.685
Wilson, M.T., Andrews, A.H., Brown, A.L. & Cordes, E.E., 2002. Axial rod growth and age estimation of the sea pen, Halipteris willemoesi Kölliker Hydrobiologia, 471, 133-142.
Wood. C., 2005. Seasearch guide to sea anemones and corals of Britain and Ireland. Ross-on-Wye: Marine Conservation Society.
Datasets
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-14
Citation
This review can be cited as:
Last Updated: 17/02/2003