Montagu's stellate barnacle (Chthamalus montagui)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Karen Riley | Refereed by | Prof. Alan J. Southward |
| Authority | Southward, 1976 | ||
| Other common names | - | Synonyms | - |
Summary
Description
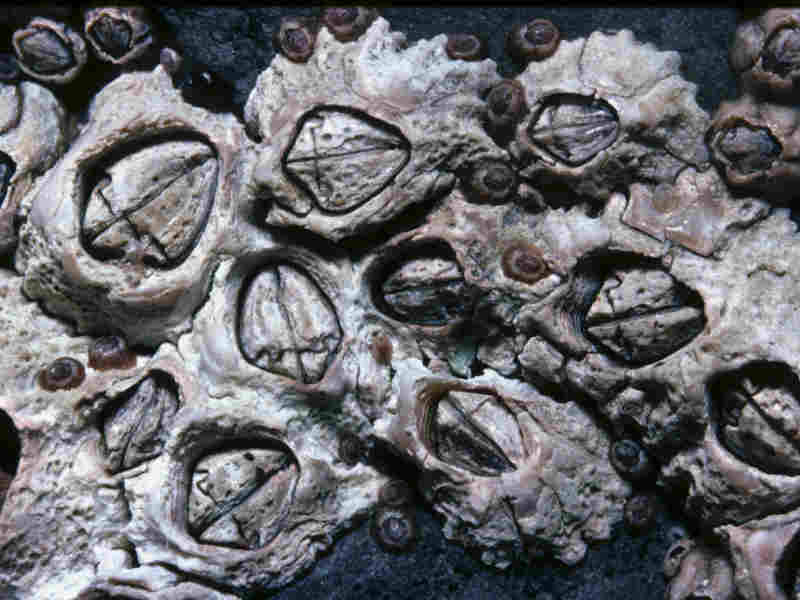
An intertidal barnacle with six coarsely ridged wall plates, a kite-shaped opercular opening, and a membranous base. The rostral plate is relatively narrow and plates are of roughly equal size. The rostral plate is not fused with rostrolaterals. The tissue inside the opercular aperture is blue (paler than in Chthamalus stellatus) with brown and black markings. Usually conical in shape, however when crowded may become tubular. It reaches a maximum diameter of approximately 14 mm, depending on habitat, food availability and level on shore.
Recorded distribution in Britain and Ireland
A warm-water species recorded on the south and west coasts of Britain as far north as Orkney and along the Scottish east coast south to Aberdeen. The Isle of Wight is its eastern limit in the English Channel. It is relatively abundant on Irish coasts.
Global distribution
Crisp et al. (1981) noted that its distribution extends through the western and eastern Mediterranean and down the north African coast to Mauritania.
Habitat
Recorded in the high to mid eulittoral zone on exposed to moderately exposed rocky shores. Its vertical distribution overlaps with that of Chthamalus stellatus and Semibalanus balanoides.
Depth range
Not relevantIdentifying features
- Shell wall of six solid plates.
- Kite-shaped operculum opening.
- The joint between the terga and scuta crosses the centre line less than one third of the way down towards the rostrum.
- Tissue inside opercular aperture is usually blue/pale blue with brown and black markings.
- Junction between terga and scuta is concave towards rostral plate.
- Shell base is membranous.
Additional information
Before 1976 Chthamalus montagui was considered a variety of Chthamalus stellatus, but in 1976 was identified as a distinct species due to differences in its vertical zonation on the shore and morphology, particularly in the shape of the opercular plates, setation of the smaller cirri, the more sheltered locations in which it was found and its different pattern of zonation (Southward, 1976).
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Arthropoda | Arthropods, joint-legged animals, e.g. insects, crustaceans & spiders |
| Family | Chthamalidae | |
| Genus | Chthamalus | |
| Authority | Southward, 1976 | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | Up to 1.4cm | ||
| Male size at maturity | |||
| Female size range | Small(1-2cm) | ||
| Female size at maturity | |||
| Growth form | |||
| Growth rate | 10 - 55 | ||
| Body flexibility | |||
| Mobility | |||
| Characteristic feeding method | Active suspension feeder, See additional information | ||
| Diet/food source | |||
| Typically feeds on | Plankton. | ||
| Sociability | |||
| Environmental position | Epifaunal | ||
| Dependency | Independent. | ||
| Supports | No information | ||
| Is the species harmful? | No | ||
Biology information
Feeding. Chthamalus stellatus / Chthamalus montagui generally feed on small plankton. They can consume diatoms, but were found not to grow under a regime dominated by diatoms (Barnes & Barnes, 1965). Normal feeding of chthamalids involves a cirral beat. This cirral beat is also noted to be a respiratory mechanism (Anderson & Southward, 1987). However, in high wave exposure they tend to hold their cirri out stiffly against the water current for a long period of time, retracting when food is captured (Crisp, 1950). Barnacles living in wave-exposed conditions may benefit from this passive suspension feeding habit where cirral beating and consequent energy expenditure are minimised (Crisp, 1950).
Rates of cirral beat decrease with age and size, but increase with temperature (Anderson & Southward, 1987). Green (1961) reported that barnacles higher up on shore had a higher cirral beat frequency than those at lower levels. However, Southward (1955; 1964(b)) found no similar trends.
Southward (1955) found that there was no cirral beat of Chthamalus stellatus / Chthamalus montagui in still water and that cirral beating was only induced at a current of approximately 10 cm/sec. The cirral beating frequency is also related to temperature, as shown by experiments by Southward (1955). Chthamalus stellatus / Chthamalus montagui barnacles kept at a temperature of 0°C did not react to touch after an hour. He also found that they remained inactive at a temperature of up to 5°C. Between 5 and 30°C there was a linear increase to 10 beats every 10 seconds. This slowly declined above 33 °C and dropped rapidly at 36 °C. Although the species resisted coma above a temperature of 40°C, all cirral beating ceased at 37.5°C.
Respiration. Sessile barnacles have a pair of gills: pleats of the mantle wall, attached to the mantle cavity (Stubbings, 1975). Rainbow (1984) also stated that the cirri might also play an important role in respiration. There is usually a slow respiratory pumping beat, with varied emergence of the cirri.
Moulting. Barnacles need to moult in order to grow. Feeding rate and temperature determine the frequency of moulting. Moulting does not take place during winter when phytoplankton levels and temperatures are low (Crisp & Patel, 1960).
Growth. Once the barnacle is fixed in place it is unable to detach again (Crisp, 1955). All species grow faster in early life and slower in later life, and chthamalids tend to become tubular when crowded (Southward & Crisp, 1965). The growth rate varies with a variety of biological and environmental factors, including current flow, orientation with respect to current, food supply, wave exposure, shore height, surface contour, and intra- or inter-specific competition. Growth in Chthamalus spp. takes place along the whole internal surface of the one-layered plates (Bourget, 1977). The growth rate for Chthamalus stellatus / Chthamalus montagui has been reported by Barnes (1956; Crisp & Bourget (1985) as between 10 - 55 µm per day (relatively slow) in the linear phase. Crisp (1950) noticed that Chthamalus stellatus / Chthamalus montagui reached a maximum size of 0.2 to 1.4 cm. Chthamalus stellatus / Chthamalus montagui was found to have a lower growth rate than many other species of barnacles (Relini, 1983). The species reached a basal diameter of 2-2.5 mm in 3 months, 3.5-4 one year later, up to 8 mm in the 2nd year of growth, but generally no more than about 5-6 mm (Relini, 1983). Sometimes a decrease in size was noticeable, due to abrasion. This low growth rate was found to be associated with a low metabolic rate, or low oxygen consumption, by Barnes & Barnes (1965).
Parasites and epizoites. Healy (1986, in O'Riordan et al., 1992) observed the parasitic isopod, Hemioniscus balani in Chthamalus stellatus and Chthamalus montagui in Ireland, although it was never present in Lough Hyne populations. However, Southward & Crisp (1954) found that although it attacks and sterilises Semibalanus balanoides individuals, it does not normally attack chthamalids on British shores.
Further Information. The dog whelk, Nucella lapillus, feeds on barnacles. The species of Chthamalus spp. are less at risk from dogwhelks due to their smaller size in comparison with Semibalanus balanoides and often higher position on the shore. Other predators which pull shells or cirri of barnacles off the rock include crabs, amphipods, shore fish such as shanny Lipophrys pholis, and sometimes herring gulls (Moore & Kitching, 1939). Another possible predator is the polychaete, Eulalia viridis (Moore & Kitching, 1939). Chthamalus spp. is also known to be displaced by Patella spp. and smothered by Mytilus spp. and algae at lower shore levels (Moore & Kitching, 1939). Empty barnacle cases provide homes for small periwinkles, small bivalves and the isopod Campecopea hirsuta (Fish & Fish, 1996). Gubbay (1983) showed that Chthamalus montagui could withstand a compressive force of 42 N and a much lower tensile force of 7.4 N, and that a membranous base adhered to the substrate better than a calcified base. In order to protect themselves from changes in temperature/desiccation and a lowering of salinity, intertidal barnacles are usually able to close their aperture tightly (Moore & Kitching, 1939)
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Enclosed coast or Embayment, Open coast |
| Biological zone preferences | Mid eulittoral, Upper eulittoral |
| Substratum / habitat preferences | Artificial (man-made), Bedrock, Large to very large boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Very strong > 6 knots (>3 m/sec.), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Exposed, Moderately exposed, Sheltered, Very exposed |
| Salinity preferences | Full (30-40 psu) |
| Depth range | Not relevant |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
Geographical distribution. Crisp et al. (1981) have described the distribution of Chthamalus stellatus and Chthamalus montagui. Chthamalus montagui occurs all around the western seaboard of Britain and all around Ireland. It is absent from part of Liverpool Bay. It occurs in Orkney but not Shetland and extends south down the east coast of Scotland to Aberdeen. Its distribution on the east coast is more or less continuous, extending from the north of Scotland, along the west coasts of Britain and along all coasts of the Irish Sea. Records detailing its worldwide distribution are limited, but it is probable that their range extends further south to Mauritania, through the western and eastern parts of the Mediterranean Sea. It is rare or absent from offshore islands. It is common in the northern Adriatic and occurs at locations in the Aegean and Black Seas.
Vertical distribution. Chthamalus montagui is dominant over Chthamalus stellatus in more sheltered sites (Southward, 1976; Crisp et al., 1981; Burrows et al., 1992). Where their distributions overlap Chthamalus montagui has a greater vertical distribution above that of Chthamalus stellatus (Burrows et al., 1992) and, while Chthamalus montagui is more common between MHWS & MHWN, Chthamalus stellatus is abundant lower down at MTL and below (Pannacciulli & Relini, 2000). Near its northern limit in Scotland Chthamalus montagui is limited to a narrow band at the top of the shore due to competition with Semibalanus balanoides (Kendall & Bedford, 1987), and the influence of lower temperatures. Poor settlement of Chthamalus spp. also usually occurs. The higher the species occurs up on the shore, the more resistant to desiccation influences they tend to be (Southward, 1955b). Physical factors such as exposure to seawater, desiccation and poor food supply limit the distribution of barnacles on the upper shore, whereas competition for space, predation and strong wave action limit the distribution at low and mid-shore levels (Pannacciulli & Relini, 2000). The distribution of Chthamalus spp. is not affected by small increases in algal cover. However, rapid increases to 100 % can lead to a massive decline in barnacle populations, declining to almost zero in a year or two (Southward, 1991). Hawkins & Hartnoll (1982) found that the lower shore level limit was controlled by the presence of algal turf.
Substratum preference. Barnacles attach themselves to hard, rough surfaces and are rarely found on chalk cliffs (Moore & Kitching, 1939). Moore & Kitching (1939) also suggested that this may be because the surface is smooth, washed away easily, or too porous (making it possible to be dried out from below).
Temperature dependence and competition. Chthamalus spp. are warm water species, with their northern limit of distribution in Britain. They tend to be more tolerant to temperature increases and desiccation than Semibalanus balanoides. Southward (1976) found that in Cornwall and Devon, where the barnacle is common, it dominates the upper half of the barnacle zone. Chthamalus spp. prefer warm temperatures, whereas Semibalanus balanoides prefers low temperatures. This is reflected by the changes in their distribution with changes in climate. For example, in the severe winter of 1962-63, Chthamalus populations declined (Southward, 1967) while Semibalanus balanoides increased, and in the temperature rise of 1988-89, the trend was reversed (Southward, 1991). Long-term trends are also evident. A decline in Chthamalus populations and an increase in Semibalanus balanoides occurred between 1962 and 1980, corresponding with a temporary decrease in sea temperatures (Southward, 1991). Since 1981 there has been a general increase in Chthamalus (Southward, 1991), maybe corresponding with gradual climate warming. Southward & Crisp (1954) noted that in 1948-51, during high temperatures in the British Isles Chthamalus dominated over Semibalanus balanoides, and during 1951-52, during lower temperatures there was a resurgence of Semibalanus balanoides. Southward (1991) noted a two-year phase lag between temperature trends and changes in barnacle abundance in Plymouth. Chthamalus spp. Are more abundant in waters where the mean temperatures are above 10 °C for several months of the year (Southward, 1955b).
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Self-fertilization |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | 1,000-10,000 |
| Generation time | 1-2 years |
| Age at maturity | 9 - 10 months |
| Season | May - August |
| Life span | 2-5 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Planktotrophic |
| Duration of larval stage | 11-30 days |
| Larval dispersal potential | Greater than 10 km |
| Larval settlement period | Insufficient information |
Life history information
Before 1976 there was no distinction between Chthamalus stellatus and Chthamalus montagui. Since 1976 the existence of two separate species was recognised (Southward, 1976). Therefore, papers pre-1976 on Chthamalus stellatus have been recorded as for both Chthamalus stellatus and Chthamalus montagui, below.
Fertilization. Sexual maturity of Chthamalus montagui was attained at a rostro-carinal diameter of 4.4.5-6.4 mm (O'Riordan et al., 1992). Chthamalus montagui is able to breed in its first year (Burrows, 1988; Southward & Crisp, 1954), after 9 to 10 months of settlement (Southward & Crisp, 1954). Sperm is activated by the oviducal gland and transferred to the oviducal sac via the penis of a neighbouring barnacle (Barnes, 1989). The barnacle penis is substantially longer than the body and is capable of searching an area around the adult to find a receptive 'functional female' (Rainbow, 1984). Barnacles generally reproduce by cross-fertilization, but Chthamalus has been shown to self-fertilize when isolated (Barnes & Barnes, 1958; Barnes, 1989); this usually occurs high up on shore. However, it has been noted that in self-fertilized individuals oviposition is delayed (Barnes & Barnes, 1958; Barnes, 1989) and the resulting eggs can be slightly abnormal and are considered less viable (Barnes, 1989). Egg masses (egg lamellae) are brooded in the mantle cavity (O'Riordan et al., 1995; Barnes, 1989).
Breeding season. Southward (1978) suggested that Chthamalus montagui breeds one to two months later than Chthamalus stellatus. However, Crisp et al. (1981) found little difference in SW Britain, with the main breeding peak in June/July and August. Throughout the breeding season most individuals produce several broods (Burrows et al., 1992; O'Riordan et al., 1992), with a small percentage of the population remaining reproductively active throughout the year (O'Riordan et al., 1995); Barnes, 1989). After the maturation of each brood ovarian and penis re-development takes place (O'Riordan et al., 1995; Barnes & Barnes, 1965; Burrows, 1988; Anderson, 1994). According to Hines (1978) temperature and food availability are the main factors controlling the duration of the breeding season and the embryonic development rate of other Chthamalus species. In fact, Burrows (1988, in Kendall & Bedford, 1987) found the onset of the breeding season to be correlated with a sea temperature of 10 °C or above (Burrows et al., 1992). Southward & Crisp (1956) noted that the interval between broods in Chthamalus stellatus and Chthamalus montagui became shorter at higher temperatures. The onset of the breeding season was noticed by Crisp (1950) to spread up the shore level over several months. Brooding in Aberystwyth was noted to be from May or June to August (Kendall & Bedford, 1987), with approximately 80 % containing a naupliar mass. Cyprid settlement occurred from late July to early September at a sea temperature of 15.3 to 18.8 °C (Kendall & Bedford, 1987). In northern Spain, the brooding period tends to be longer, between April and early October, with 30 % containing a naupliar mass (Kendall & Bedford, 1987). The breeding period, period of larval settlement and density of recruits are all reduced near the northern limits of its distribution. Crisp (1950) suggested that for Chthamalus montagui and Chthamalus stellatus in the United Kingdom, breeding commenced earlier with decreasing longitude and easterly longitude. Breeding of Chthamalus stellatusand Chthamalus montagui usually takes place earlier in the year in continental Europe than in the British Isles (Relini & Matricardi, 1979; Relini, 1983; Miyares, 1986, all in O'Riordan et al., 1995). In the Mediterranean, the breeding season usually occurs in July and August (Mizrahi & Achituv, 1990, in O'Riordan et al., 1995). Experiments by O'Riordan et al. (1995) showed that in their first year Chthamalus stellatus and Chthamalus montagui breed once or more, and more than once thereafter.
Embryonic development. In both Chthamalus stellatus and Chthamalus montagui it took approximately 23 days for embryos to develop completely in vivo at 15°C (Burrows et al., 1992; Burrows, 1988, in Kendall & Bedford, 1987). Chthamalus montagui will only breed if temperatures exceed 15°C (Patel & Crisp, 1960).
Recruitment and lifespan. Towards the northern limits of the species distribution annual recruitment is low (Kendall & Bedford, 1987) and individuals have an increased longevity (Lewis, 1964). The normal lifespan of Chthamalus stellatus / Chthamalus montagui at mid-shore level is considered to be approximately 2-3 years (Southward & Crisp, 1956). However, growth is more rapid and the mortality rate is greater lower down on the shore (Southward & Crisp, 1956).
Fecundity. Burrows et al. (1992) found that the number of eggs per brood for Chthamalus montagui ranged between 1,030 and 1,803 in Britain, depending on body size and weight. It was also noted by (Burrows et al., 1992) that the fecundity generally increased with lower shore levels colonized, with estimations of one to two broods per year at high shore levels, two to over three at mid-shore levels, and over two to over four at low shore levels.
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceBarnacles are permanently attached to hard rough surfaces. Therefore, loss of substratum due to activities such as spoil dumping or land claim will result in loss of individuals in the area. If suitable substrata remains within the area, colonization of juvenile barnacles is possible. Intolerance is assessed as high. Recoverability is likely to be moderate (see Additional Information section below). | High | Moderate | Moderate | High |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceChthamalus stellatus / Chthamalus montagui have been shown to be relatively unaffected by smothering by oil. Monterosso (1930) showed experimentally that the species can survive complete smothering by petroleum jelly for approximately two months, by respiring anaerobically. Complete smothering caused by the Torrey Canyon oil spill yielded similar results; A few Semibalanus balanoides died, yet Chthamalus stellatus / Chthamalus montagui seemed unaffected, while at Booby's bay more than 90 % had managed to clear an opening in the oil film (Smith, 1968). Although oil had very little effect on individuals, it is likely that smothering by sediment can clog breathing apparatus. Recruitment to the smothered area will also be reduced. Therefore intolerance is assessed as intermediate. Recoverability is likely to be high (see Additional Information section below). | Intermediate | High | Low | Moderate |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceBarnacles are likely to be able to tolerate a slight increase in siltation. A large increase in siltation to 100 mg/l for one month is may block breathing apparatus and impose an energetic cost of cleaning the gills. Intolerance is therefore, assessed as low. Recoverability is likely to be very high as feeding and respiratory structures are likely to be clear of particles within a short space of time. | Low | Very high | Very Low | Low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceA decrease in siltation is unlikely to affect Chthamalus montagui populations. | Tolerant | Not relevant | Not sensitive | Not relevant |
Desiccation [Show more]Desiccation
EvidenceChthamalus montagui is a warm water species, with its northern limit of distribution in Britain. It tends to be more tolerant to desiccation than Semibalanus balanoides. The higher the species occurs up on the shore, the more resistant to desiccation influences they tend to be (Southward, 1955b). Cracks and crevices offer further protection from desiccation. Southward (1958) reported an internal temperature of 28.8 °C in an air temperature of 13.7 °C.Chthamalids are prevented from growing higher up the shore due to their desiccation tolerance. Therefore, an increase in the level of desiccation would cause a depression in the upper limit of the species vertical distribution. A decrease in the level of desiccation may elevate their upper limit. Therefore, intolerance is assessed as low. Recoverability is likely to be very high (see Additional Information section below). | Low | Very high | Very Low | Moderate |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceAccording to Hines (1978) temperature and food availability are the main factors controlling the duration of the breeding season and the embryonic development rate in other species of Chthamalus. With an increase in emergence, the period of time covered by the water would decrease, and the time available for feeding and breeding would also decrease. This is likely to reduce the growth rate and annual recruitment. There is also likely to be a shift downwards on the shore due to competition with Semibalanus balanoides. Intolerance is assessed as intermediate. Recoverability is likely to be high (see Additional Information section below). | Intermediate | High | Low | Moderate |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceBarnacle populations are likely to be tolerant of an decrease in emergence. According to Hines (1978) temperature and food availability are the main factors controlling the duration of the breeding season and the embryonic development rate in other species of Chthamalus. With a decrease in the emergence regime, the feeding time and breeding possibilities are likely to increase. Adults of Chthamalus stellatus/ Chthamalus montagui can survive permanent submersion (Barnes, 1953). However, competition between Semibalanus balanoides is likely to play an important role in the changes in the species distribution. It is likely that the distribution of Chthamalus montagui will move further up the shore, with no noticeable difference in the range. Intolerance is assessed as low. Recoverability is likely to be very high (see Additional Information section below). | Low | Very high | Very Low | High |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceAn increase in water flow rate is likely to lead to higher growth rates and annual recruitment. Intolerance is assessed as low. Recoverability is likely to be very high (see Additional Information section below). | Low | High | Low | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceA decrease in the water flow rate is likely to lead to a decrease in growth rate and annual recruitment. Intolerance is assessed as low. Recoverability is likely to be very high (see Additional Information section below). | Low | Very high | Very Low | Low |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceChthamalus montagui would be favoured by an increase in temperature based on the following information:
| Tolerant* | Not relevant | Not sensitive* | High |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceDuring the severe winter of 1962-63, over the majority of the species range, chthamalids were able to withstand the cold. However, greater mortalities were noted to occur a month or two after the coldest weather (Crisp, 1964). Chthamalid populations declined while Semibalanus balanoides increased (Southward, 1967). A decline in Chthamalus sp. populations and an increase in Semibalanus balanoides occurred between 1951 and 1975, corresponding with a decrease in sea temperatures (Southward, 1991). Southward & Crisp (1954) noted during 1951-52, during lower temperatures there was a resurgence of Semibalanus balanoides. (Southward, 1991) noted a two year phase lag between temperature trends and changes in barnacle abundance in Plymouth.Chthamalus sp. is more abundant in waters where the mean temperatures are above 10 °C for several months of the year (Southward, 1955b). According to Hines (1978) temperature and food availability are the main factors controlling the duration of the breeding season and the embryonic development rate. In fact, Burrows (1988, in O'Riordan et al., 1995) found the onset of the breeding season to be correlated with a sea temperature of 10 °C or above (Burrows et al., 1992). Southward & Crisp (1956) noted that the interval between broods in Chthamalus stellatus and Chthamalus montagui became shorter at higher temperatures. Chthamalus montagui will only breed in temperatures above 15 degrees C (Patel & Crisp, 1960). A decrease in temperature is therefore likely to result in greater mortality of Chthamalus species, and a resurgence in Semibalanus balanoides. Recruitment is also likely to decline. Intolerance is assessed as high. Recoverability is likely to be low (see Additional Information section below). | High | Low | High | High |
Increase in turbidity [Show more]Increase in turbidity
EvidenceBarnes & Barnes (1968) found that in high suspended solids and low salinity there was a decrease in the number of eggs per brood of Chthamalus stellatus / Chthamalus montagui. Fecundity in protected areas such as harbours is usually lower, possibly due to increased turbidity (Barnes, 1989). Intolerance is assessed as intermediate. Recoverability is likely to be moderate (see Additional Information section, below.) | Intermediate | Moderate | Moderate | Moderate |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceA decrease in turbidity is likely to lead to an increase in the quantity of flagellates available in the water column. Chthamalus montagui is therefore assessed as tolerant*. | Tolerant* | Not relevant | Not sensitive* | Low |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceChthamalus montagui colonizes exposed to moderately exposed rocky shores. An increase in wave exposure is therefore unlikely to affect the species. | Tolerant | Not relevant | Not sensitive | Not relevant |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceChthamalus montagui colonizes exposed to moderately exposed rocky shores. A decrease in wave exposure below 'moderately exposed' is likely to result in a proportion of the population dying. A decrease in the level of wave exposure may also cause a shift in the community towards fucoid algae, which prevent barnacle larvae settlement. Intolerance is assessed as intermediate. Recoverability is likely to be moderate (see Additional Information section below). | Intermediate | Moderate | Moderate | High |
Noise [Show more]Noise
EvidenceBarnacles are unlikely to be affected by noise. | Tolerant | Not relevant | Not sensitive | Not relevant |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceBarnacles are unlikely to be affected by visual presence. | Tolerant | Not relevant | Not sensitive | Not relevant |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceCracks and crevices offer protection from some abrasion but the majority of barnacles are on open rock surfaces and liable to be crushed by abrasive forces such as cobbles moving in wave action or vessel strandings. Small abrasive forces, such as erosion from suspended sediment, has been noted to cause a decrease in barnacle size (Relilni, 1983). On a larger scale, Gubbay (1983) showed that Chthamalus montagui could withstand a compressive force of 42 newtons (N) and a much lower tensile force of 7.4 N, perhaps equivalent to trampling pressure. Therefore, intolerance is assessed as intermediate. Recoverability is likely to be high (see additional information below). | Intermediate | High | Low | Low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceOnce the barnacle is fixed in place it is unable to attach again (Crisp, 1955). Intolerance to displacement is therefore assessed as high. Recoverability is likely to be moderate (see Additional Information section below). | High | Moderate | Moderate | High |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceBarnacles have a low resilience to chemicals such as dispersants, dependant on the concentration and type of chemical involved (Holt et al., 1995). They are less intolerant than some species (e.g. Patella vulgata) to dispersants (Southward & Southward, 1978). In areas which where large amounts of detergents had been used, there was much greater mortality, and in Kynance cove the population was wiped out completely (Smith, 1967). However, the barnacle population suffered indirectly as a result of the mass mortality of grazers. The resultant bloom of algae, and growth of fucoids, within 6 months, grew over and killed surviving barnacles (Hawkins & Southward, 1992). Intolerance to synthetic chemicals is assessed as intermediate. Recoverability is likely to be high (see Additional Information section, below). | Intermediate | Moderate | Moderate | Very low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceBarnacles accumulate heavy metals and store them as insoluble granules. No information is available as to the effects of heavy metals on Chthamalus montagui, but a larger amount of information was found with respect to a barnacle from the same family, Semibalanus balanoides. It is possible that sensitivities to heavy metals may be similar in both species. Clarke (1947) investigated the intolerance of Semibalanus balanoides to copper, mercury, zinc and silver. He found that 90 percent of barnacles died when held in 0.35 mg/l Cu carbonate for two days. Zinc, mercury and silver killed 90 percent of barnacles in two days at concentrations of 32 mg/l, 1 mg/l and 0.4 mg/l respectively. Pyefinch & Mott (1948) recorded median lethal concentrations of 0.32 mg/l copper and 0.36 mg/l mercury over 24 hours for this species. Barnacles may tolerate fairly high level of heavy metals in nature, for example they are found in Dulas Bay, Anglesey, where copper reaches concentrations of 24.5 µg/l, due to acid mine waste (Foster et al., 1978). Therefore, intolerance to heavy metals is assessed as low. Recoverability is likely to be high (see Additional Information section, below). | Low | High | Low | Very low |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceChthamalus stellatus/ Chthamalus montagui have been shown to be relatively unaffected by smothering by oil (Southward & Southward, 1978; Smith, 1967). Monterosso (1930) showed experimentally that the species can survive complete smothering by petroleum jelly for approximately two months, by respiring anaerobically. Complete smothering caused by the Torrey Canyon oil spill yielded similar results; A few Semibalanus balanoides died, yet Chthamalus stellatus / Chthamalus montagui seemed unaffected (Southward & Southward, 1978; Smith, 1968), while at Booby's Bay more than 90 % had managed to clear an opening in the oil film. On further examination these individuals were found to be in good condition, with no oil present in the gut (Smith, 1967).However, detergents used to clean up the oil lead to a decline in Chthamalus sp. populations. In areas which where large amounts of detergents had been used, there was much greater mortality, and in Kynance cove the population was wiped out completely (Smith, 1967).Therefore, intolerance is assessed as low. Recoverability is likely to be high (see Additional Information section, below). | Low | High | Low | Moderate |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficientinformation. | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceLittle data exists on the effects of increased nutrients on barnacles. A slight increase in nutrient levels may be beneficial for barnacles by promoting the growth of flagellates. However, Holt et al. (1995) predict that smothering by ephemeral green algae is a possibility under eutrophic conditions. Therefore, intolerance to nutrient levels is assessed as intermediate. Recoverability is likely to be high (see Additional Information section, below). | Intermediate | High | Low | Low |
Increase in salinity [Show more]Increase in salinity
EvidenceChthamalids only occur in full salinity water, therefore an increase in salinity is unlikely to exert a noticeable effect. The species is recorded as not intolerant of an increase in salinity. | Tolerant | Not relevant | Not sensitive | Not relevant |
Decrease in salinity [Show more]Decrease in salinity
EvidenceBarnacles are able to acclimate over a number of days to reduced salinity (Rainbow, 1984; Moore & Kitching, 1939; Foster, 1970). However, the acclimatisation, or closing of the opercular plate is also associated with anaerobiosis and low metabolic activity (Barnes et al., 1963). Barnes & Barnes (1965) found that in high suspended solids and low salinity there was a decrease in the number of eggs per brood of Chthamalus stellatus / Chthamalus montagui. If salinities decrease below 21 psu all cirral activity of barnacles that are normally associated with full salinity coastal waters, ceases (Foster, 1971). Therefore, intolerance to a decrease in salinity is assessed as high. Recoverability is likely to be high (see Additional Information section, below). | High | High | Moderate | Moderate |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceSouthward (1955) conducted experiments on the relationship of cirral activity in Chthamalus stellatus / Chthamalus montagui, connected with feeding and respiration, to decreased oxygenation, by passing nitrogen through the water at 6 ml per minute at 13 °C. He found that in all cases a decrease in oxygen concentration lead to a decrease in cirral activity and that, after 15 minutes, the mean cirral beat had decreased from 3.1 to 2.9 beats per second. After 30 minutes exposure, cirral beat had completely ceased and the barnacle remained inactive. It was further observed that the scuta and terga remained slightly open with the cirri often protruding.Barnacles have to obtain oxygen from the water through their cirri including by cirral beating in still water. Since cirri stop beating in response to lowered oxygen levels, it seems likely that intolerance will be high. Therefore, intolerance to oxygen levels is assessed as high. Recoverability is likely to be high (see Additional information section, below). | High | High | Moderate | Moderate |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceHealy (1986, in O'Riordan et al., 1992) has observed the parasitic isopod, Hemioniscus balani in Chthamalus stellatus and Chthamalus montagui in Ireland, although it was never present in Lough Hyne. However, Southward & Crisp (1954) found that, although it attacks and sterilises Semibalanus balanoides individuals, it does not attack chthamalids, at least not in the British Isles. Therefore, intolerance is assessed as intermediate. Recoverability is likely to be high (see Additional Information section, below). | Intermediate | High | Low | Low |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceThe Australasian barnacle Elminius modestus was introduced to British waters on ships during the second world war. The species does well in estuaries and bays, where it can displace Semibalanus balanoides and Chthamalus montagui. The native species are not displaced completely because they out-compete Elminius on exposed shores (Raffaelli & Hawkins, 1999). Intolerance to the introduction of non-native species is assessed as intermediate. Recoverability is likely to be high (see Additional information section, below). | Intermediate | High | Low | Moderate |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceNot relevant. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceCollection of intertidal algae may damage barnacles by abrasion from trampling. Intolerance to the extraction of other species is assessed as low. Recoverability is likely to be high (see Additional information section, below). | Low | High | Low | Low |
Additional information
Chthamalus montagui is able to breed in its first year (Burrows, 1988, in O'Riordan et al., 1995; Southward & Crisp, 1954), nine to ten months after settlement (Southward & Crisp, 1954). Throughout the breeding season most individuals produce several broods (Burrows et al., 1992; O'Riordan et al., 1992), with a small percentage of the population remaining reproductively active throughout the year (O'Riordan et al., 1995; Barnes, 1989).Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
-none-Bibliography
Anderson, D.T. & Southward, A.J., 1987. Cirrial activity of barnacles. In Barnacle biology, (ed. A.J. Southward), pp. 135-174. Rotterdam: A.A. Balkema. [Crustacean Issues no. 5.]
Anderson, D.T., 1994. Barnacles. Structure, function, development and evolution. Melbourne: Chapman & Hall.
Barnes, H. & Barnes, M., 1958. Further observations on self-fertilisation in Chthamalus sp. Ecology, 39, 550.
Barnes, H. & Barnes, M., 1965. Egg size, nauplius size, and their variation with local, geographical and specific factors in some common cirripedes. Journal of Animal Ecology, 34, 391-402.
Barnes, H., 1953. The effect of lowered salinity on some barnacle nauplii. Journal of Animal Ecology, 22, 328-330.
Barnes, H., 1956. The growth rate of Chthamalus stellatus (Poli). Journal of the Marine Biological Association of the United Kingdom, 35, 355-361.
Barnes, H., Finlayson, D.M. & Piatigorsky, J., 1963. The effect of desiccation and anaerobic conditions on the behaviour, survival and general metabolism of three common cirripedes. Journal of Animal Ecology, 32, 233-252.
Barnes, M., 1989. Egg production in Cirripedia. Oceanography and Marine Biology: an Annual Review, 27, 91-166.
Barnes, M., 1992. The reproductive periods and condition of the penis in several species of common cirripedes. Oceanography and Marine Biology: an Annual Review, 30, 483-525.
Bassindale, R., 1964. British Barnacles. London: The Linnean Society of London.[Synopses of the British Fauna, no. 14.]
Bokn, T.L., Moy, F.E. & Murray, S.N., 1993. Long-term effects of the water-accommodated fraction (WAF) of diesel oil on rocky shore populations maintained in experimental mesocosms. Botanica Marina, 36 (4), 313-319. DOI https://doi.org./10.1515/botm.1993.36.4.313
Bourget, E., 1977. Shell structure in sessile barnacles. Le Naturaliste Canadien, 104, 281-323.
Burrows, M.T., 1988. The comparative biology of Chthamalus Stellatus (Poli) and Chthamalus montagui Southward. PhD thesis, University of Manchester. 318pp.,
Burrows, M.T., Hawkins, S.J. & Southward, A.J., 1992. A comparison of reproduction in co-occurring chthamalid barnacles, Chthamalus stellatus (Poli) and Chthamalus montagui Southward. Journal of Experimental Marine Biology and Ecology, 160, 229-249.
Burrows, M.T., Hawkins, S.J. & Southward, A.J., 1999. Larval development of the intertidal barnacles Chthamalus stellatus and Chthamalus montagui. Journal of the Marine Biological Association of the United Kingdom, 79, 93-101.
Clarke, G.L., 1947. Poisoning and recovery in barnacles and mussels. Biological Bulletin, Marine Biological Laboratory, Woods Hole, 92, 73-91.
Crisp, D.J. & Barnes, H., 1954. The orientation and distribution of barnacles at settlement with particular reference to surface contour. Journal of Animal Ecology, 23, 142-162.
Crisp, D.J. & Bourget, E., 1985. Growth in Barnacles. Advances in Marine Biology, 22, 199-244.
Crisp, D.J. & Patel, B.S., 1960. The moulting cycle in Balanus balanoides (L.). Biological Bulletin, Marine Biological Laboratory, Woods Hole, 118, 31-47.
Crisp, D.J. (ed.), 1964. The effects of the severe winter of 1962-63 on marine life in Britain. Journal of Animal Ecology, 33, 165-210.
Crisp, D.J., 1950. Breeding and distribution of Chthamalus stellatus. Nature (London), 166, 311-312.
Crisp, D.J., 1955. The behaviour of barnacle cyprids in relation to water movement over a surface. Journal of Experimental Biology, 32, 569-590.
Crisp, D.J., 1974. Factors influencing the settlement of marine invertebrate larvae. In Chemoreception in Marine Organisms, Chapter 5 (ed. P.T. Grant & A.M. Mackie), pp. 177-265. London: Academic Press.
Crisp, D.J., Southward, A.J. & Southward, E.C., 1981. On the distribution of the intertidal barnacles Chthamalus stellatus, Chthamalus montagui and Euraphia depressa. Journal of the Marine Biological Association of the United Kingdom, 61, 359-380.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Foster, B.A., 1970. Responses and acclimation to salinity in the adults of some balanomorph barnacles. Philosophical Transactions of the Royal Society of London, Series B, 256, 377-400.
Foster, B.A., 1971b. On the determinants of the upper limit of intertidal distribution of barnacles. Journal of Animal Ecology, 40, 33-48.
Foster, P., Hunt, D.T.E. & Morris, A.W., 1978. Metals in an acid mine stream and estuary. Science of the Total Environment, 9, 75-86.
Green, J., 1961. A biology of Crustacea. London: H.F. & G. Witherby Ltd. 180 pp.
Gubbay, S., 1983. Compressive and adhesive strengths of a variety of British barnacles. Journal of the Marine Biological Association of the United Kingdom, 63, 541-555.
Hawkins, S.J. & Hartnoll, R.G., 1982. Settlement patterns of Semibalanus balanoides in the Isle of Man (1977-1981). Journal of Experimental Marine Biology and Ecology, 62, 271-283.
Hawkins, S.J. & Southward, A.J., 1992. The Torrey Canyon oil spill: recovery of rocky shore communities. In Restoring the Nations Marine Environment, (ed. G.W. Thorpe), Chapter 13, pp. 583-631. Maryland, USA: Maryland Sea Grant College.
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hines, A.H., 1978. Reproduction in three species of intertidal barnacle from central California. Biological Bulletin, Marine Biological Laboratory, Woods Hole, 154, 262-281.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Kendall, M.A. & Bedford, M.L., 1987. Reproduction and recruitment of the barnacle Chthamalus montagui at Aberystwyth (mid-Wales). Marine Ecology Progress Series, 38, 305-308.
Lewis, J.R., 1964. The Ecology of Rocky Shores. London: English Universities Press.
Mathieson, A.C., Neefus, C.D. & Penniman, C.E., 1983. Benthic ecology in an estuarine tidal rapid. Botanica Marina, 26, 213-230.
Monterosso, B., 1930. Studi cirripedologici. VI. Sul comportamento di Chthamalus stellatus in diverse condizioni sperimentali. Atti Accad. Naz. Lincei Rc., 9, 501-504.
Moore, H.B. & Kitching, J.A., 1939. The biology of Chthamalus stellatus (Poli). Journal of the Marine Biological Association of the United Kingdom, 23, 521-541.
Moore, P.G. & Seed, R. (ed.), 1985. The Ecology of Rocky Coasts. London: Hodder and Stoughton Publ.
Mortlock, A.M., Fitzsimons, J.T.R. & Kerkaut, G.A., 1984. The effects of farnesol on the late stage nauplius and free swimming cypris larvae of Elminius modestus. Comparative Biochemistry and Physiology, 78A, 345-357.
O'Riordan, R.M., Myers, A.A. & Cross, T.F., 1992. Brooding in the intertidal barnacles Chthamalus stellatus (Poli) and Chthamalus montagui (Southward) in south-western Ireland. Journal of Experimental Marine Biology and Ecology, 164, 135-145.
O'Riordan, R.M., Myers, A.A. & Cross, T.F., 1995. The reproductive cycles of Chthamalus stellatus (Poli) and Chthamalus montagui (Southward) in south-western Ireland. Journal of Experimental Marine Biology and Ecology, 190, 17-38.
O'Riordan, R.M., Myers, A.A., McGrath, D., Delany, J. & Power, A-M., 1999. The sizes at settlement in natural populations of the cyprids of Chthamalus montagui and Chthamalus stellatus. Journal of the Marine Biological Association of the United Kingdom, 79, 365-366.
Pannacciulli, F.G. & Relini, G., 2000. The vertical distribution of Chthamalus montagui and Chthamalus stellatus (Crustacea, Cirripedia) in two areas of the NW Mediterranean Sea. Hydrobiologia, 426, 105-112.
Patel, B. & Crisp, D. J., 1960. The influence of temperature on the breeding and the moulting activities of some warm-water species of operculate barnacles. Journal of the Marine Biological Association of the United Kingdom, 36, 667-680.
Patel, B. & Crisp, D.J., 1960. Rates of development of the embryos of several species of barnacles. Physiology and Zoology, 33, 104-119.
Pyefinch, K.A. & Mott, J.C., 1948. The sensitivity of barnacles and their larvae to copper and mercury. Journal of Experimental Biology, 25, 276-298.
Raffaelli, D.G. & Hawkins, S.J., 1999. Intertidal Ecology 2nd edn.. London: Kluwer Academic Publishers.
Rainbow, P.S., 1984. An introduction to the biology of British littoral barnacles. Field Studies, 6, 1-51.
Relini, G., 1983. Remarks on the ecology of Chthamalids in the Ligurian Sea. Rapp. P.-V. Reun. Ciesm., 28, 273-275.
Smith, J.E. (ed.), 1968. 'Torrey Canyon'. Pollution and marine life. Cambridge: Cambridge University Press.
Southward, A., 1978. Marine life and Amoco Cadiz. New Scientist, 79, 174-176
Southward, A.J. & Crisp, D.J., 1954. The distribution of certain intertidal animals around the Irish coast. Proceedings of the Royal Irish Academy, 57B, 1-29.
Southward, A.J. & Crisp, D.J., 1956. Fluctuations in the distribution and abundance of intertidal barnacles. Journal of the Marine Biological Association of the United Kingdom, 35, 211-229.
Southward, A.J. & Crisp, D.J., 1965. Activity rhythms of barnacles in relation to respiration and feeding. Journal of the Marine Biological Association of the United Kingdom, 45, 161-185.
Southward, A.J. & Southward, E.C., 1978. Recolonisation of rocky shores in Cornwall after use of toxic dispersants to clean up the Torrey Canyon spill. Journal of the Fisheries Research Board of Canada, 35, 682-706.
Southward, A.J., 1955. On the behaviour of barnacles. I. The relation of cirral and other activities to temperature. Journal of the Marine Biological Association of the United Kingdom, 34, 403-432.
Southward, A.J., 1955b. On the behaviour of barnacles II. The influence of habitat and tidal level on cirral activity. Journal of the Marine Biological Association of the United Kingdom, 34, 423-433.
Southward, A.J., 1958. Note on the temperature tolerances of some intertidal animals in relation to environmental temperatures and geographical distribution. Journal of the Marine Biological Association of the United Kingdom, 37, 49-56.
Southward, A.J., 1964b. The relationship between temperature and rhythmic cirral activity in some Cirripedia considered in connection with their geographical distribution. Helgolander Wissenschaftliche Meeresuntersuchungen, 10, 391-403.
Southward, A.J., 1967. Recent changes in abundance of intertidal barnacle in south-west England: a possible effect of climatic deterioration. Journal of the Marine Biological Association of the United Kingdom, 47, 81-85.
Southward, A.J., 1976. On the taxonomic status and distribution of Chthamalus stellatus (Cirripedia) in the north-eastern Atlantic region: with a key to the common intertidal barnacles of Britain. Journal of the Marine Biological Association of the United Kingdom, 56, 1007-1028.
Southward, A.J., 1991. Forty years of changes in species composition and population density of barnacles on a rocky shore near Plymouth. Journal of the Marine Biological Association of the United Kingdom, 71, 495-513.
Stubbings, H.G., 1975. Balanus balanoides. Liverpool: Liverpool University Press.
Tighe-Ford, D.J., 1977. Effects of juvenile hormone analogues on larval metamorphosis in the barnacle Elminius modestus Darwin. Journal of Experimental Marine Biology and Ecology, 26, 163-176.
Walker, G., 1995. Larval settlement: Historical and future perspectives. In New Frontiers in Barnacle Evolution, (ed. F.R. Schram & J.T. Hoeg). Rotterdam: A.A. Balkema. [Crustacean Issues 10]
Willemsen, P.R., Overbeke, K. & Suurmond, A., 1998. Repetitive testing of TBTO, Sea-nine 211 and farnesol using Balanus amphitrite (Darwin) cypris larvae: variability in larval sensitivity. Biofouling, 12, 133-147.
Wu, R.S.S., Lam, P.K.S. & Zhou, B.S., 1997. Effects of two oil dispersants on phototaxis and swimming behaviour of barnacle larvae. Hydrobiologia, 352, 9-16.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Manx Biological Recording Partnership, 2017. Isle of Man wildlife records from 01/01/2000 to 13/02/2017. Occurrence dataset: https://doi.org/10.15468/mopwow accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2018. Isle of Man historical wildlife records 1995 to 1999. Occurrence dataset: https://doi.org/10.15468/lo2tge accessed via GBIF.org on 2018-10-01.
Merseyside BioBank., 2018. Merseyside BioBank (unverified). Occurrence dataset: https://doi.org/10.15468/iou2ld accessed via GBIF.org on 2018-10-01.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-07-31
Outer Hebrides Biological Recording, 2018. Invertebrates (except insects), Outer Hebrides. Occurrence dataset: https://doi.org/10.15468/hpavud accessed via GBIF.org on 2018-10-01.
South East Wales Biodiversity Records Centre, 2018. SEWBReC Myriapods, Isopods, and allied species (South East Wales). Occurrence dataset: https://doi.org/10.15468/rvxsqs accessed via GBIF.org on 2018-10-02.
Yorkshire Wildlife Trust, 2018. Yorkshire Wildlife Trust Shoresearch. Occurrence dataset: https://doi.org/10.15468/1nw3ch accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 28/01/2002