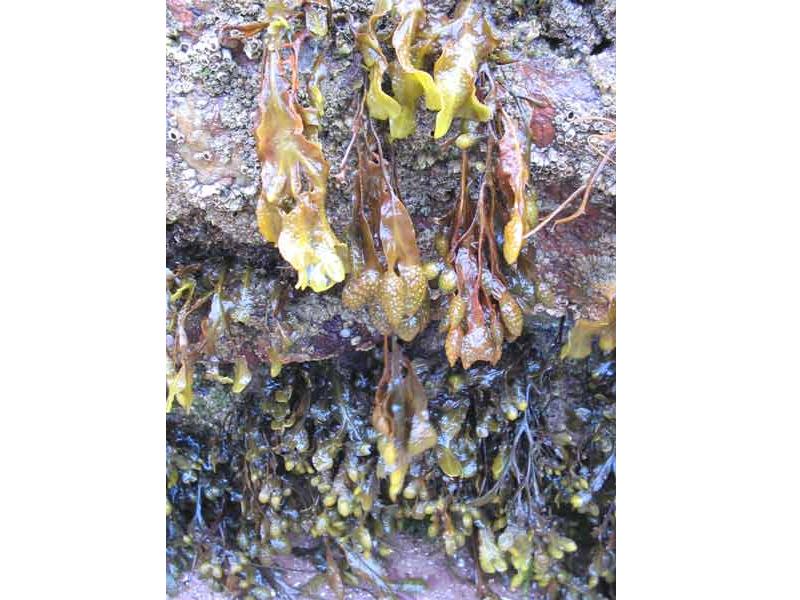
Spiral wrack (Fucus spiralis)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Nicola White | Refereed by | Dr Graham Scott |
| Authority | Linnaeus, 1753 | ||
| Other common names | - | Synonyms | - |
Summary
Description
An intertidal brown seaweed, found on the high shore. It grows up to 40 cm long, without air bladders and lives for up to 4 years. The species can tolerate a high level of desiccation. Fronds have a characteristic ridge along the edge of the receptacles.
Recorded distribution in Britain and Ireland
All coasts of Britain and IrelandGlobal distribution
Iceland, Norway, Denmark, Netherlands, UK, Ireland, Atlantic coast of France, Spain, Morocco, Azores, East coast of America from New Jersey to Nova Scotia and isolated reports in the Northern Pacific.Habitat
Fucus spiralis attaches to rocky substrata on sheltered to moderately exposed shores. It lives on the upper shore below the zone of Pelvetia canaliculata and above Fucus vesiculosus and Ascophyllum nodosum.Depth range
Not relevantIdentifying features
- Frond with smooth margin.
- Prominent midrib.
- Without air bladders.
- Frond often twisted.
- Round reproductive bodies at ends of branches, which are almost round in outline and surrounded by a narrow rim of sterile frond.
Additional information
A number of discrete forms of this species have been recorded. In the UK, a diminutive form Fucus spiralis nanus is relatively common.
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Ochrophyta | Brown and yellow-green seaweeds |
| Class | Phaeophyceae | |
| Order | Fucales | |
| Family | Fucaceae | |
| Genus | Fucus | |
| Authority | Linnaeus, 1753 | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | Up to 40cm | ||
| Male size at maturity | 3cm | ||
| Female size range | 3cm | ||
| Female size at maturity | |||
| Growth form | Foliose | ||
| Growth rate | 1.1cm/month | ||
| Body flexibility | |||
| Mobility | |||
| Characteristic feeding method | Autotroph | ||
| Diet/food source | |||
| Typically feeds on | |||
| Sociability | |||
| Environmental position | Epifloral | ||
| Dependency | Independent. | ||
| Supports | None | ||
| Is the species harmful? | No | ||
Biology information
Fucus spiralis spends up to 90 percent of the time out of the water. It can tolerate a high level of desiccation, being able to survive 70 to 80 percent water loss. Distinct varieties of Fucus spiralis have been recognised, such as Fucus spiralis forma nanus, which is a dwarf form present on exposed shores. Fucus spiralis also hybridises with Fucus vesiculosus providing considerable difficulty in identification.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Strait or Sound, Sea loch or Sea lough, Ria or Voe, Estuary |
| Biological zone preferences | Lower littoral fringe |
| Substratum / habitat preferences | Bedrock, Cobbles, Large to very large boulders, Small boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Moderately exposed, Sheltered, Very sheltered |
| Salinity preferences | Full (30-40 psu), Reduced (18-30 psu), Variable (18-40 psu) |
| Depth range | Not relevant |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
Fucus spiralis favours rocks with many cracks and fissures, which probably provide some protection for developing zygotes and adult plants. It can extend into estuaries up to the 10 psu isohaline. The presence or absence of suitable substrata is considered to be one of the most important factors determining the distribution of Fucus spiralis.Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Permanent (synchronous) hermaphrodite |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | No information |
| Generation time | 2-5 years |
| Age at maturity | 2 years |
| Season | July - August |
| Life span | 2-5 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Not relevant |
| Duration of larval stage | No information |
| Larval dispersal potential | No information |
| Larval settlement period | Insufficient information |
Life history information
Fucus spiralis is hermaphroditic. Receptacles are initiated during late January to February, gametes discharged during July and August, and the receptacles shed by November, although exact timing of reproduction depends on location and the form of the plant. Young plants usually reach a length of 8 to 10 cm or more before they form receptacles. Reproduction usually begins before or during the second years growth. Vegetative recruitment occurs by the formation of new fronds from existing holdfasts. This form of reproduction is important in existing stands of the population, whereas recruitment by eggs is more important in disturbed areas or in areas where germlings are protected e.g. rock crevices.Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceFucus spiralis is permanently attached to the substratum so would be removed upon substratum loss. The species has been observed to readily recruit to cleared areas (Holt et al., 1997) so recovery rates are expected to be high. | High | High | Moderate | High |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceThe effects of smothering would depend on the state of the tide when the factor occurred. If smothering happened when the plant was emersed, all surfaces of the plant would be buried under the sediment preventing photosynthesis. If smothering occurred while the plant was immersed some of the plant would escape burial allowing the plant continue photosynthesis. The species has been observed to readily recruit to cleared areas (Holt et al., 1997) so recovery rates are expected to be high. | High | High | Moderate | Moderate |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceIncreased siltation would cover some of the frond surfaces reducing photosynthesis and growth rates. Upon return to normal siltation levels the growth rate would be quickly restored. | Low | Very high | Very Low | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidenceFucus spiralis can tolerate desiccation until the water content has been reduced to 10-20% (Lüning, 1990). If water is lost beyond this critical level irreversible damage occurs. As the plant lives at the upper limit of it's physiological tolerance the plant cannot tolerate increased desiccation and the upper limit of the species distribution on the shore would become depressed. Decreased desiccation may allow the plant to grow further up the shore and may result in the species being competitively displaced by faster growing species. The species has been observed to readily recruit to cleared areas (Holt et al., 1997) so recovery rates are expected to be high. | High | High | Moderate | Moderate |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceFucus spiralis can tolerate an emersion period of 1-2 days. If emersion lasted for longer than this, the plant would suffer from desiccation and nutrient stress and the upper limit of the species distribution on the shore would become depressed. A reduction in the period of emersion may result in the species being competitively displaced by faster growing species and may allow Fucus spiralis to grow further up the shore. Recovery would be high because the species has been observed to rapidly recruit to cleared areas of the shore. | High | High | Moderate | Moderate |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceAn increase in water flow rate may cause some of the plants to be torn off the substratum. Decreases in water flow rate are unlikely to have any effect. Fucus spiralis has been observed to readily recruit to cleared areas (Holt et al., 1997) so recovery rates are expected to be high. | Intermediate | High | Low | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceFucus spiralis can tolerate temperatures from -0.5 to 28 °C. The species is well within it's temperature range in the UK. Decreases in temperature are unlikely to have any effect because the species extends into northern Norway where water temperatures are cooler. Increase in temperature may be beneficial because the optimum temperature for growth of the species is 15 degrees C (Lüning, 1990). However the species showed suffered some damage during the unusually hot summer of 1983 when temperatures were on average 8.3 degrees C higher than normal (Hawkins & Hartnoll, 1985). | Low | Not relevant | NR | High |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceThe species would only be affected by turbidity when it is covered in water, due to a reduction in the light available for photosynthesis. However, Fucus spiralis spends up to 90 percent of it's time out of the water and can photosynthesise effectively in air, so it would not be affected significantly by a change in turbidity. | Low | High | Low | Moderate |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceFucus spiralis lives on sheltered to moderately exposed shores. Increases in wave exposure beyond this would result in plants and germlings being torn off the substratum or mobilisation of substratum with the plants attached. Decreases in waves exposure are unlikely to have any effect, because the species occurs in very sheltered conditions. Fucus spiralis has been observed to readily recruit to cleared areas (Holt et al., 1997) so recovery rates are expected to be high. | High | High | Moderate | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidenceSeaweeds have no known mechanisms for perception of noise. | Tolerant | Not relevant | Not sensitive | Not relevant |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceSeaweeds have no known mechanism for visual perception. | Tolerant | Not relevant | Not sensitive | Not relevant |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceAbrasion may kill germlings and damage the fronds of established seaweeds. Fucoids are intolerant of abrasion from human trampling, which has been shown to reduce the cover of seaweeds on a shore (Holt et al., 1997). Germlings are probably particularly intolerant of this factor. Fucus spiralis has been observed to readily recruit to cleared areas (Holt et al., 1997) so recovery rates are expected to be high. | Intermediate | High | Low | Low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceFucus spiralis is permanently attached to the substratum and would not be able to re-establish itself if removed. The species has been observed to readily recruit to cleared areas (Holt et al., 1997) so recovery rates are expected to be high. | High | High | Moderate | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceAdult fucoid algae accumulate heavy metals and are generally fairly robust in the face of chemical pollution (Holt et al., 1997). However, germlings appear to be intolerant of heavy metal pollution. Copper retarded the growth rate of Fucus spiralis sporelings at concentrations greater than 5.8 µg/l and caused permanent damage in sporelings exposed to concentrations of 12.24 µg/l for 10 days (Bond et al., 1999). The species has been observed to readily recruit to cleared areas (Holt et al., 1997) so recovery rates are expected to be high. | Intermediate | High | Low | Moderate |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceFucoids generally show limited intolerance to oils (Holt et al., 1997). However, Fucus spiralis disappeared from heavily oiled shores some months after the Amoco Cadiz oil spill. The species suffered less than Pelvetia canaliculata but more than fucoids further down the shore, probably due to it's position high on the shore, which means the oil can be present on the algae for a long time before being washed off (Floc'h & Diouris, 1980). | High | High | Moderate | Low |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceDecreases in nutrient concentration may decrease growth rate in Fucus spiralis. A slight increase in nutrient concentration may enhance growth rates but high concentrations of nutrients would lead to overgrowth of the plants by ephermeral green algae. However, Fucus spiralis is reported to be more common than other fucoids in the sewage polluted inner part of the Oslofjord, Norway (Fletcher, 1996). Recovery rate should be high because cleared areas of the shore are rapidly recruited by this species. | Intermediate | High | Low | Low |
Increase in salinity [Show more]Increase in salinity
EvidenceFucus spiralis can experimentally tolerate salinities of 3 to 34 psu, but it is only found in estuaries down to 10 psu so it may be affected by this factor. | Intermediate | Very high | Low | Moderate |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceReduced oxygenation is unlikely to have an effect on the algae as it produces its own oxygen by photosynthesis. However, no studies have been found to confirm this. | No information | Not relevant | No information | Not relevant |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceFucus spiralis rapidly recruits to cleared areas (Holt et al., 1997) so would recover reasonably quickly from extraction of 50 percent of the area. | Intermediate | High | Low | Moderate |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Additional information
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Fucus spiralis does not support encrusting or sessile epifauna although the amphipod Hyale and the littorinids Littorina obtusata and Littorina saxatilis occur amongst fronds which provide shelter from desiccation. A range of epiphytes may also grow on the fronds.Bibliography
Anderson, C.I.H. & Scott, G.W., 1998. The occurrence of distinct morphotypes within a population of Fucus spiralis. Journal of the Marine Biological Association of the United Kingdom, 78, 1003-1006.
Bond, P.T., Brown, M.T., Moate, R.M., Gledhill, M., Hill, S.J. & Nimmo, M., 1999. Arrested development in Fucus spiralis (Phaeophyceae) germlings exposed to copper. European Journal of Phycology, 34, 513-521.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Fletcher, R.L., 1996. The occurrence of 'green tides' - a review. In Marine Benthic Vegetation. Recent changes and the Effects of Eutrophication (ed. W. Schramm & P.H. Nienhuis). Berlin Heidelberg: Springer-Verlag. [Ecological Studies, vol. 123].
Floc'h, J. H. & Diouris, M., 1980. Initial effects of Amoco Cadiz oil on intertidal algae. Ambio, 9, 284-286.
Hardy, F.G. & Guiry, M.D., 2003. A check-list and atlas of the seaweeds of Britain and Ireland. London: British Phycological Society
Hawkins, S.J. & Hartnoll, R.G., 1985. Factors determining the upper limits of intertidal canopy-forming algae. Marine Ecology Progress Series, 20, 265-271.
Hazlett, A. & Seed, R., 1976. A study of Fucus spiralis and its associated fauna in Strangford Lough, Co. Down. Proceedings of the Royal Irish Academy, 76, 607-618.
Holt, T.J., Hartnoll, R.G. & Hawkins, S.J., 1997. The sensitivity and vulnerability to man-induced change of selected communities: intertidal brown algal shrubs, Zostera beds and Sabellaria spinulosa reefs. English Nature, Peterborough, English Nature Research Report No. 234.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Niemeck, R.A. & Mathieson, A.C., 1976. An ecological study of Fucus spiralis. Journal of Experimental Marine Biology and Ecology, 24, 33-48.
Norton, T.A. (ed.), 1985. Provisional Atlas of the Marine Algae of Britain and Ireland. Huntingdon: Biological Records Centre, Institute of Terrestrial Ecology.
Robertson, B.L., 1985. Reproductive ecology and canopy structure of Fucus spiralis (L.) Botanica Marina, 30, 475-482.
Scott, G.W., Shaw, J.H., Hull, S.L., Pickaert, C. & Burlak, A.M., 1999. Some implications of plant size in monotypic and polytypic populations of Fucus spiralis. Journal of the Marine Biological Association of the United Kingdom, 80, 359-360.
Vernet, P. & Harper, J.L., 1980. The costs of sex in seaweeds. Biological Journal of the Linnean Society, 13, 129-138.
Datasets
Bristol Regional Environmental Records Centre, 2017. BRERC species records recorded over 15 years ago. Occurrence dataset: https://doi.org/10.15468/h1ln5p accessed via GBIF.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2014. Occurrence dataset: https://doi.org/10.15468/erweal accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2016. Occurrence dataset: https://doi.org/10.15468/146yiz accessed via GBIF.org on 2018-09-27.
Kent Wildlife Trust, 2018. Biological survey of the intertidal chalk reefs between Folkestone Warren and Kingsdown, Kent 2009-2011. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Manx Biological Recording Partnership, 2017. Isle of Man wildlife records from 01/01/2000 to 13/02/2017. Occurrence dataset: https://doi.org/10.15468/mopwow accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2018. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset: https://doi.org/10.15468/aru16v accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2018. Isle of Man historical wildlife records 1995 to 1999. Occurrence dataset: https://doi.org/10.15468/lo2tge accessed via GBIF.org on 2018-10-01.
Merseyside BioBank., 2018. Merseyside BioBank (unverified). Occurrence dataset: https://doi.org/10.15468/iou2ld accessed via GBIF.org on 2018-10-01.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-07-12
Outer Hebrides Biological Recording, 2018. Non-vascular Plants, Outer Hebrides. Occurrence dataset: https://doi.org/10.15468/goidos accessed via GBIF.org on 2018-10-01.
Royal Botanic Garden Edinburgh, 2018. Royal Botanic Garden Edinburgh Herbarium (E). Occurrence dataset: https://doi.org/10.15468/ypoair accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. SEWBReC Algae and allied species (South East Wales). Occurrence dataset: https://doi.org/10.15468/55albd accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. Dr Mary Gillham Archive Project. Occurance dataset: http://www.sewbrec.org.uk/ accessed via NBNAtlas.org on 2018-10-02
Yorkshire Wildlife Trust, 2018. Yorkshire Wildlife Trust Shoresearch. Occurrence dataset: https://doi.org/10.15468/1nw3ch accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 29/05/2008