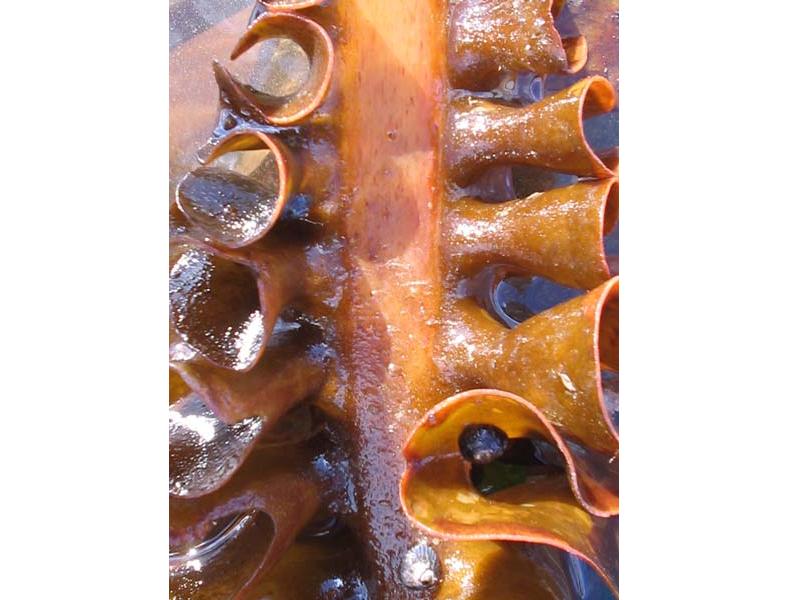
Furbelows (Saccorhiza polyschides)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Nicola White | Refereed by | This information is not refereed |
| Authority | (Lightfoot) Batters, 1902 | ||
| Other common names | - | Synonyms | Saccorhiza bulbosa (Lightfoot) Batters, 1902, Laminaria polyschides |
Summary
Description
Saccorhiza polyschides is kelp species with a distinctive large warty holdfast and a flattened stipe with a frilly margin. The stipe is twisted at the base and widens to form a large flat lamina, which is divided into ribbon-like sections. The species is an annual, and very fast growing. It is opportunistic and colonizes available hard substrata in the sublittoral.
Recorded distribution in Britain and Ireland
Recorded from the all coasts of Britain and Ireland, but absent from Northumberland to the Solent.
Global distribution
Furbelows' recorded distribution extends from Ghana northwards along the European coastline, with the most northerly recorded location at Rorvik, Norway. It has also been reported in the Eastern Mediterranean extending to the Greek coastline, and Italy.
Habitat
Saccorhiza polyschides grows from extreme low water springs to a depth of 35 m. It normally attaches to rocks but is occasionally found loose-lying on small stones or shells. It can form dense stands in sheltered areas and can tolerate strong currents.
Depth range
0 -35mIdentifying features
- Stipe flat, broad with conspicuously frilled margin and twisted at base.
- Wide frond, without midrib and divided into ribbon-like sections.
- Large bulbous holdfast with warty appearance.
- Up to 4 m in length.
Additional information
No text entered
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Ochrophyta | Brown and yellow-green seaweeds |
| Class | Phaeophyceae | |
| Order | Tilopteridales | |
| Family | Phyllariaceae | |
| Genus | Saccorhiza | |
| Authority | (Lightfoot) Batters, 1902 | |
| Recent Synonyms | Saccorhiza bulbosa (Lightfoot) Batters, 1902Laminaria polyschides | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Moderate density | ||
| Male size range | |||
| Male size at maturity | |||
| Female size range | Large(>50cm) | ||
| Female size at maturity | |||
| Growth form | Forest | ||
| Growth rate | 145mm/week | ||
| Body flexibility | |||
| Mobility | |||
| Characteristic feeding method | Autotroph | ||
| Diet/food source | Photoautotroph | ||
| Typically feeds on | |||
| Sociability | |||
| Environmental position | Epilithic | ||
| Dependency | Independent. | ||
| Supports | No information | ||
| Is the species harmful? | No | ||
Biology information
- Saccorhiza polyschides is a fast growing, annual and opportunistic species. The obvious plant is a gender-less sporophyte which grows up to 4 m long and may grow at 2 m a month at the peak of the growth season in late spring. The large sporophytes are present on the shore from May until winter. In autumn they commence fruiting and start to decay, leaving behind the bulbous holdfast, which remains on the shore until it is washed off in late winter.
- The unusual holdfast of Saccorhiza polyschides is formed from a hollow bulbous growth above the sapling holdfast which expands to overwhelm it, sending out secondary haptera to attach to the substratum.
- The shape of the frond varies with the degree and nature of water movement. In sites of low water current plants produce broad undivided fronds, while those in areas of strong currents have long deeply divided fronds. Plants from wave exposed locations have short fronds divided into few sections. Experiments have shown that these variations are due to phenotypic rather than genotypic variation (Norton, 1978).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Offshore seabed, Open coast, Ria or Voe, Sea loch or Sea lough, Strait or Sound |
| Biological zone preferences | Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Bedrock, Cobbles, Large to very large boulders, Pebbles, Small boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Very strong > 6 knots (>3 m/sec.), Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Extremely sheltered, Moderately exposed, Sheltered, Ultra sheltered, Very sheltered |
| Salinity preferences | Full (30-40 psu) |
| Depth range | 0 -35m |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
- Saccorhiza polyschides colonizes abraded surfaces such as sand-scoured rocks or boulders that are mobile in winter and is characteristic of disturbed substrata.
- Plants grow to a maximum depth of 35 metres in Cornwall. The lower depth limit of the plants may be controlled by grazing from the sea urchin Echinus esculentus. When urchins have been removed, the lower limit of Saccorhiza polyschides has been found to extend by 3 m.
- The species is not found in areas of reduced salinity. Lowered salinity reduces the rate of development and growth is irreversibly inhibited below 9 psu. The species competes for space with Laminaria hyperborea and the upper limit of Saccorhiza polyschides is related to the lower limit of Laminaria hyperborea. Where Laminaria hyperborea is absent the species may extend up to the extreme low water springs mark.
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Alternation of generations |
| Reproductive frequency | Semelparous or monotely |
| Fecundity (number of eggs) | No information |
| Generation time | <1 year |
| Age at maturity | 8-14 months |
| Season | October - May |
| Life span | <1 year |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Spores (sexual / asexual) |
| Duration of larval stage | < 1 day |
| Larval dispersal potential | 100 -1000 m |
| Larval settlement period | Insufficient information |
Life history information
- Saccorhiza polyschides has a typical Laminarian life history in which a macroscopic diploid sporophyte alternates with a microscopic haploid gametophyte.
- The species is an annual. Sporophytes typically have a lifespan of less than 10 months. However, plants produced late in the season may overwinter and live for 14-16 months.
- The base of the lamina, the stipe frills and the bulb are covered in unilocular sporangia, which produce zoospores by meiosis. Each sporangia contains 128 zoospores. The flagellated zoospores are about 5 microns in diameter and possess an eyespot which makes them strongly phototactic. The zoospores may be transported at least 200 m from the parent and they loose their flagella after 24 hrs and settle on the available substrata. 75% of the zoospores settle on the substrata with 24 hours.
- The zoospores develop into microscopic dioecious gametophytes. Gametophytes take the form of unicellular or filamentous structures. The male gametophytes are more branched than the females and have more numerous, smaller and paler cells. These become fertile in under 10 days in optimal conditions. Male gametophytes release motile sperm that fertilize eggs of female gametophytes, the resultant zygote develops into the new sporophyte.
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceSaccorhiza polyschides is permanently attached to the substratum so will be removed upon substratum loss. Experiments have shown that Saccorhiza polyschides colonizes cleared areas of the substratum within 26 weeks. However, if clearance takes place in August, when no spores of the species are released, the substratum may become colonized by red algae potentially blocking colonization by Saccorhiza polyschides (Kain, 1975). | High | High | Moderate | Moderate |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceSmothering could reduce light availability and therefore lower growth rates of the sporophyte but would not damage the plant. The microscopic sporophytes and gametophytes are likely to be more intolerant and if smothered growth would be inhibited, except on vertical surfaces where development appears to be unaffected (Norton, 1978). | Low | Immediate | Not sensitive | Low |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceSiltation is unlikely to affect the adult sporophytes but microscopic juvenile stages may be harmed. Norton (1978) observed that when spores settled on silt they continued development but failed to form attachments and would be easily washed off. Silt settling out on already attached spores prevented the formation of gametophytes and sporophytes. However, Birkett et al. (1998b), states that the species is found in areas of siltation and Santos (1993) observed that Saccorhiza polyschides is abundant in areas of high siltation, so the species may tolerate siltation. Recovery should be high because experiments have shown that Saccorhiza polyschides colonizes cleared areas of the substratum within 26 weeks. However, if clearance takes place in August, when no spores of the species are released the substratum may become colonized by red algae (Kain, 1975). | Intermediate | High | Low | Low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidenceThe species is intolerant of desiccation. Norton (1970) observed that when sporophytes were exposed to air by an extreme low water springs on a hot summers day, they rapidly dried out and died. An increase in the level of desiccation would depress the upper limit of the species distribution. Recovery should be high because experiments have shown that Saccorhiza polyschides colonizes cleared areas of the substratum within 26 weeks and it has a very fast growth rate. However, if clearance takes place in August, when no spores of the species are released the substratum may become colonized by red algae (Kain, 1975). | High | High | Moderate | Moderate |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceSaccorhiza polyschides is intolerant of aerial exposure. Norton (1970) observed that when sporophytes were exposed to air by an extreme low water spring tide on a hot summers day, they rapidly dried out and died. An increase in the period of emersion would depress the upper limit of the species distribution. Recovery should be high because experiments have shown that Saccorhiza polyschides colonizes cleared areas of the substratum within 26 weeks and it has a very fast growth rate. However, if clearance takes place in August, when no spores of the species are released the substratum may become colonized by red algae (Kain, 1975). | High | High | Moderate | Moderate |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceSaccorhiza polyschides occurs in a wide range of water flow rates. It is found in areas of high water flow such as rapids in Lough Hyne (Ine), Ireland, but also grows in almost stationary water, where it can form extensive loose-lying populations in the absence of turbulence (Norton, 1978). The species is therefore unlikely to be affected by a change in water flow. | Low | High | Low | Moderate |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceThe minimum temperature required for growth and reproduction of Saccorhiza polyschides is 5 degrees C and the maximum temperature is 23 degrees C. The 'northern lethal boundary' of the species occurs where the temperature falls below 4 degrees C for a period of 2 months and the southern lethal boundary occurs where temperatures rise above 25 degrees C for more than a few weeks (Hoek van den, 1982). The species is in the middle of its geographic range in the UK so is unlikely to be affected by a change of 2 degrees C for a year. However, a change in 5 degrees may put the species outside its lethal limits damaging the plant. Recovery should be high because the species has a fast growth rate and rapidly colonizes cleared areas of the substratum. | Intermediate | High | Low | Moderate |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceLight penetration influences the depth at which kelps can grow. An increase in turbidity would reduce light available for photosynthesis, lower growth rates and result in a decrease of the maximum depth at which it could grow. A reduction in the turbidity levels would allow Saccorhiza polyschides to grow at greater depths but the upper limit of the species distribution would be depressed due to increased competition with Laminaria hyperborea. On return to normal turbidity levels the growth rate and depth distribution would be quickly resumed | Low | High | Low | Low |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceSaccorhiza polyschides is found at all wave exposures (Hawkins & Jones, 1992) so is not likely to be intolerant of this factor. Increases in wave exposure which cause substrata to be mobilized and for abrasion to occur might be favourable to Saccorhiza. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidenceSeaweeds have no known mechanism for the perception of noise. | Tolerant | Not relevant | Not sensitive | Moderate |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceSeaweeds have no known mechanism for visual perception. | Tolerant | Not relevant | Not sensitive | Moderate |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceThe fronds of Saccorhiza polyschides could be damaged by abrasion and gametophytes could be crushed. A passing scallop dredge or anchor is likely to rip off the plant and its holdfast, and remove a proportion of the population. Therefore, intolerance has been assessed as intermediate. However, the species has a fast growth rate, settles and grows to full size annually and, therefore, recovers very quickly from disturbance. | Intermediate | High | Low | Moderate |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceTransplantation experiments have shown that plants can be transplanted to other sites with the rocks that they are attached to, with no adverse effects (Norton, 1978). The species could not tolerate displacement if the attachment to the rock was broken. | Low | Very high | Very Low | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceThe effects of chemicals on Saccorhiza polyschides have not been studied. Laminaria hyperborea, a related species of kelp, is thought to be fairly robust in terms of chemical pollution (Holt et al., 1995). Both species contain alginates which seem capable of storing chemicals in inert forms. However, it is likely that the gametophytes and very young sporophytes are more intolerant. Hopkin & Kain (1978) observed that growth of gametophytes and very young sporophytes of Laminaria hyperborea was inhibited at low levels of atrazine, sodium pentachlorophenate and phenol. | Intermediate | High | Low | Very low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceThe effects of heavy metals on Saccorhiza polyschides have not been studied. Laminaria hyperborea, a related species of kelp, is thought to be fairly robust in terms of chemical pollution (Holt et al., 1995). Both species contain alginates which seem capable of storing metals in inert forms. However, it is likely that the gametophytes and very young sporophytes are more intolerant. Hopkin & Kain (1978) observed that growth of gametophytes and very young sporophytes of Laminaria hyperborea was inhibited at low levels of mercury, cadmium, copper and zinc. | Intermediate | High | Low | Very low |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceA number of workers have reported little effect of oil on sublittoral kelp, due to the lack of penetration of oil into the water column (Holt et al., 1995). Drew et al. (1967) recorded that the kelp forest escaped undamaged after the 'Torrey Canyon' oil spillage. Kelp may also be protected by the mucilaginous slime which covers the frond, by preventing damage from coating by oil (Birkett et al., 1998b). No studies have been carried out specifically on the impact on Saccorhiza polyschides but the alga is probably tolerant of this factor. Recovery rates would be high due to the fast growth rate of the species and its ability to rapidly colonize cleared areas of the substratum. | Low | High | Low | Very low |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceNutrients are required for algal growth. A slight increase in nutrient levels may enhance growth rates but a large increase may have a detrimental effect. Eutrophication could reduce the lower depth limit of the species distribution by reducing light penetration through an increase in turbidity. There may also be increased competition with mussels for available substratum space and plants may be overgrown by ephemeral green algae (Birkett et al.,1998b). However, Saccorhiza polyschides has a very fast growth rate and can probably effectively compete with these species, so it is only has low intolerance to this factor and recovery rates would be high. | Low | High | Low | Very low |
Increase in salinity [Show more]Increase in salinity
EvidenceSaccorhiza polyschides is not found in areas of reduced salinity. In culture, lowered salinities have been found to reduce growth rate and development is irreversibly inhibited below 9 psu (Norton & South, 1969), so the species is regarded as highly intolerant of this factor. Recovery rates should be high because the species has a high growth rate and quickly colonizes cleared areas of the substratum (Kain, 1975). | High | High | Moderate | High |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceThe effect of low oxygen levels on kelp is poorly studied. Saccorhiza polyschides can grow in almost stationary water (Norton, 1978) and can generate its own oxygen by photosynthesis, so it is likely to tolerate changes in this factor. The species can quickly recover from disturbance as it has a fast growth rate and rapidly colonizes cleared areas of the substratum (Kain, 1975). | Low | High | Low | Very low |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceThe Japanese kelp Undaria pinnatifida has recently spread to the south coast of England from Brittany, where it was introduced for aquaculture. It is thought that Undaria may compete with Saccorhiza polyschides (Birkett et al., 1998b). The potential introduction of Macrocystis spp. from America could have an enormous impact on native kelps due to the very fast growth rate of the species. | Intermediate | High | Low | Low |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceSaccorhiza polyschides has a fast growth rate and rapidly colonizes cleared areas of the substratum, so it would be able to quickly recover from harvesting. | Intermediate | Very high | Low | Moderate |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceGrazing urchins, such as Echinus esculentus and Paracentrotus lividus are important in determining the lower depth limit of Saccorhiza polyschides. The removal of these species may enable Saccorhiza to grow at greater depths, for example Kain & Jones (1966) found that removal of grazing urchins at Port Erin, Isle of Man, enabled Saccorhiza polyschides to extend its depth range by 3 m. Likewise, the upper limit of the species distribution is related to Laminaria hyperborea and the extraction of this species would enable Saccorhiza to grow in shallower water. Where beds of Laminaria hyperborea or Laminaria digitata have been cleared, they are usually replaced by Saccorhiza polyschides (Norton, 1970). | Tolerant* | Not relevant | Not sensitive* | Moderate |
Additional information
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Saccorhiza polyschides is not harvested for alginate production at present, but may be of interest in the future because of its high growth rate. The culture of this species has been undertaken experimentally, without successful results (Guiry & Blunden, 1991). The sporophytes of Saccorhiza polyschides support rich communities of epifauna and epiflora. However, no species are known to be confined to Saccorhiza polyschides. The holdfasts of Saccorhiza polyschides are known to shelter large animals such as large polychaetes, squat lobsters and fish which shelter inside the bulbous holdfast, while amphipods, brittle stars and polychaetes occur in the space between the base of the bulb and the rock surface to which it is attached (McKenzie & Moore, 1981). The composition of the epifauna and epiflora varies with the degree of water current that the plant is exposed to (Ebling et al., 1948). The fronds are grazed by urchins such as Echinus esculentus and Paracentrotus lividus, and the blue-rayed limpet Patella pellucidaBibliography
Birkett, D.A., Maggs, C.A., Dring, M.J. & Boaden, P.J.S., 1998b. Infralittoral reef biotopes with kelp species: an overview of dynamic and sensitivity characteristics for conservation management of marine SACs. Natura 2000 report prepared by Scottish Association of Marine Science (SAMS) for the UK Marine SACs Project., Scottish Association for Marine Science. (UK Marine SACs Project, vol VI.), 174 pp. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/reefkelp.pdf
Ebling, F.J., Kitching, J.A., Purchon, R.D. & Bassingdale, R., 1948. The ecology of Lough Ine rapids with special reference to water currents. 2. The fauna of the Saccorhiza canopy. Journal of Animal Ecology, 17, 223-244.
Guiry, M.D. & Blunden, G., 1991. Seaweed Resources in Europe: Uses and Potential. Chicester: John Wiley & Sons.
Guiry, M.D. & Nic Dhonncha, E., 2002. AlgaeBase. World Wide Web electronic publication http://www.algaebase.org,
Hardy, F.G. & Guiry, M.D., 2003. A check-list and atlas of the seaweeds of Britain and Ireland. London: British Phycological Society
Hawkins, S. J. & Jones, H. D., 1992. Rocky Shores. London: Immel.
Hopkin, R. & Kain, J.M., 1978. The effects of some pollutants on the survival, growth and respiration of Laminaria hyperborea. Estuarine and Coastal Marine Science, 7, 531-553.
Kain, J.M. & Jones, N.S., 1966. Algal colonisation after removal of Echinus. Proceedings of the International Seaweed Symposium, 8, 139-140.
Kain, J.M., 1975a. Algal recolonization of some cleared subtidal areas. Journal of Ecology, 63, 739-765.
McKenzie, J.D. & Moore, P.G., 1981. The microdistribution of animals associated with the bulbous holdfasts of Saccorhiza polyschides (Phaeophyta). Ophelia, 20, 201-213.
Norton, T.A. & Burrows, E.M., 1969. Studies on marine algae of the British Isles. 7. Saccorhiza polyschides (Lightf.) Batt. British Phycological Journal, 4, 19-53.
Norton, T.A. & South, G.R., 1969. Influence of reduced salinity on the distribution of two laminarian algae. Oikos, 20, 320-326
Norton, T.A., 1970. Synopsis of biological data on Saccorhiza polyschides. FAO Fisheries Synopsis, No. 83, 1-35.
Norton, T.A., 1978. The factors influencing the distribution of Saccorhiza polyschides in the region of Lough Ine. Journal of the Marine Biological Association of the United Kingdom, 58, 527-536.
Santos, R., 1993. A multivariate study of biotic and abiotic relationships in a subtidal algal stand. Marine Ecology Progress Series, 94, 181-190.
Van den Hoek, C., 1982. The distribution of benthic marine algae in relation to the temperature regulation of their life histories. Biological Journal of the Linnean Society, 18, 81-144.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Manx Biological Recording Partnership, 2017. Isle of Man wildlife records from 01/01/2000 to 13/02/2017. Occurrence dataset: https://doi.org/10.15468/mopwow accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2018. Isle of Man historical wildlife records 1995 to 1999. Occurrence dataset: https://doi.org/10.15468/lo2tge accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2022. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset:https://doi.org/10.15468/aru16v accessed via GBIF.org on 2024-09-27.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-08
Outer Hebrides Biological Recording, 2018. Non-vascular Plants, Outer Hebrides. Occurrence dataset: https://doi.org/10.15468/goidos accessed via GBIF.org on 2018-10-01.
Royal Botanic Garden Edinburgh, 2018. Royal Botanic Garden Edinburgh Herbarium (E). Occurrence dataset: https://doi.org/10.15468/ypoair accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 29/05/2008