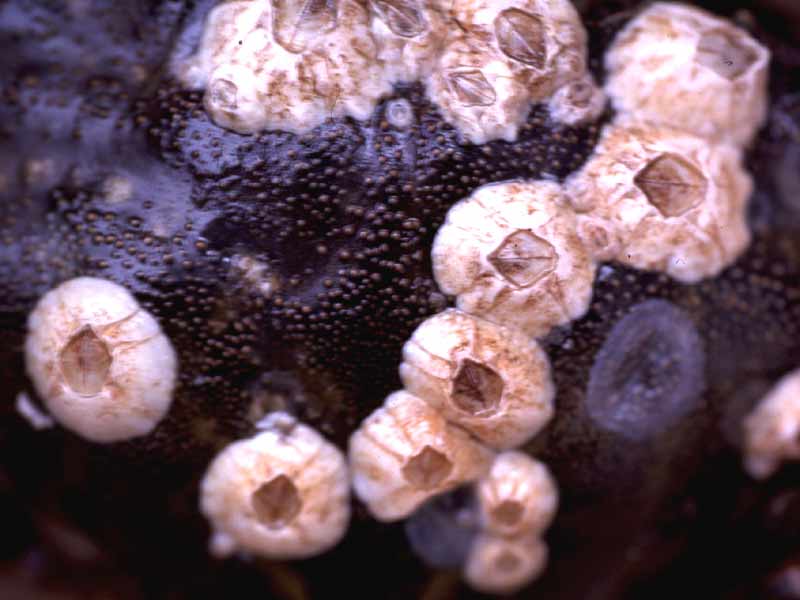
Wrinkled barnacle (Balanus crenatus)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Nicola White | Refereed by | Prof. Alan J. Southward |
| Authority | Bruguière, 1789 | ||
| Other common names | Crenate barnacle | Synonyms | - |
Summary
Description
Balanus crenatus is one of the most common sublittoral barnacles in Britain. It has six shell plates and grows up to 25 mm in diameter. The upper edge of the shell plates are usually toothed and the shell is inclined to one end when viewed in profile. It usually lives for around 18 months.
Recorded distribution in Britain and Ireland
All coasts of Britain & Ireland, and offshore in the North Sea and Celtic Sea.Global distribution
Northeast Atlantic from the Arctic to the west coast of France as far south as Bordeaux; east and west coasts of North America and Japan.Habitat
Balanus crenatus is primarily a sublittoral species that can sometimes be found under stones or overhangs on the lower shore. Balanus crenatus colonizes cobbles, shells, bedrock, molluscs and artificial substrata. It is found at a wide range of wave exposures and it can tolerate salinities as low as 14 psu.Depth range
Data deficientIdentifying features
- Shell wall of 6 grey white plates.
- Up to 25 mm diameter.
- Opercular aperture a broad diamond shape.
- Upper edge of shell plates toothed.
- Shell inclined to one end when viewed in profile.
- Shell base calcareous.
- Tissue inside opercular aperture with yellow and purple stripes.
Additional information
No text entered
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Arthropoda | Arthropods, joint-legged animals, e.g. insects, crustaceans & spiders |
| Family | Balanidae | |
| Genus | Balanus | |
| Authority | Bruguière, 1789 | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Moderate density | ||
| Male size range | |||
| Male size at maturity | |||
| Female size range | Small(1-2cm) | ||
| Female size at maturity | |||
| Growth form | |||
| Growth rate | 4.4mm/month | ||
| Body flexibility | None (less than 10 degrees) | ||
| Mobility | |||
| Characteristic feeding method | Active suspension feeder, Passive suspension feeder | ||
| Diet/food source | |||
| Typically feeds on | Zooplankton and other organic particles of a suitable size, such as detritus and phytoplankton. | ||
| Sociability | |||
| Environmental position | Epifaunal | ||
| Dependency | Independent. | ||
| Supports | None | ||
| Is the species harmful? | Data deficient | ||
Biology information
Balanus crenatus has a calcareous base, while Semibalanus balanoides has a membranous base.
Feeding
Balanus crenatus feeds by extending thoracic appendages called cirri out from the shell to filter zooplankton from the water. In the absence of any current, the barnacle rhythmically beats the cirri. When a current is present Balanus crenatus holds the cirri fully extended in the current flow. Barnacles feed most during spring and autumn when plankton levels are highest. Little if any feeding takes place during winter, when barnacles rely on stored food reserves. Feeding rate is important in determining the rate of growth.
Moulting
Barnacles need to moult in order to grow. Frequency of moulting is determined by feeding rate and temperature. Moulting does not take place during winter when phytoplankton levels and temperatures are low.
Size:
Balanus crenatus is hermaphroditic and grows up to 25mm in diameter.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast, Offshore seabed, Strait or Sound, Sea loch or Sea lough, Ria or Voe, Estuary, Enclosed coast or Embayment |
| Biological zone preferences | Lower eulittoral, Lower infralittoral, Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Artificial (man-made), Bedrock, Cobbles, Gravel / shingle, Large to very large boulders, Pebbles, Small boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Very strong > 6 knots (>3 m/sec.), Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Exposed, Extremely exposed, Extremely sheltered, Moderately exposed, Sheltered, Very exposed, Very sheltered |
| Salinity preferences | Full (30-40 psu), Low (<18 psu), Reduced (18-30 psu), Variable (18-40 psu) |
| Depth range | Data deficient |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
Balanus crenatus is a widespread species that occurs at quite high latitudes in the Arctic. It colonizes a wide range of substrata, attaching to any hard substrata, molluscs and their dead shells (Southward, pers. comm.), often as an initial colonizing species. Densely packed colonies occur particularly in areas exposed to strong tidal streams where few other epifauna survive. It can also be found attached to carapaces of the Norway lobster or Dublin Bay prawn (Nephrops norvegicus) and other crustaceans.Balanus crenatus may have been misidentified as Solidobalanus fallax in shallow waters lying to the south of the UK. The deep water record of Gruvel (noted in Southward, 1998) is an error (Southward, pers. comm.). Balanus crenatus and Solidobalanus fallaxcolonize different substrates and also occur in different temperatures. Solidobalanus fallax occurs in warmer water on shells, false corals, seaweeds and other soft substrata, including plastic bags and synthetic netting (Southward, pers. comm.).
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Permanent (synchronous) hermaphrodite |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | No information |
| Generation time | <1 year |
| Age at maturity | 4 months |
| Season | February - September |
| Life span | 1-2 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Lecithotrophic |
| Duration of larval stage | 11-30 days |
| Larval dispersal potential | Greater than 10 km |
| Larval settlement period | Insufficient information |
Life history information
- Balanus crenatus is an obligate cross-fertilizing hermaphrodite. Nauplii larvae are released from the barnacle between February and September, with peaks in April and late summer when phytoplankton levels are highest. However, release is not synchronised with the spring algal bloom, unlike Semibalanus balanoides.
- Nauplii larvae are planktotrophic and develop in the surface waters. They pass through six nauplii stages before eventually developing into a cyprid larva. Cyprid larvae are specialised for settlement. They drift and swim in the plankton before selecting a suitable substratum for settlement and metamorphosis. Peak settlement occurs in April and declines until October. Metamorphosis usually takes place within 24 hours of settlement.
- Barnacles grow rapidly except in winter. April-settled individuals may release larvae the same July and reach full size before their first winter. Individuals that settled later reach maximum size by the end of spring the following year (Rainbow, 1984).
- Balanus crenatus has a lifespan of 18 months (Barnes & Powell, 1953). Growth rate varies greatly with the degree of current flow and the presence of silt. Balanus crenatus populations attached to Nephrops norvegicus grew only 2 mm in 4 months, whereas populations on rafts grew at 0.2 mm per day. This reduction in growth in epizoic populations is attributed to the higher presence of silt and the reduction in water currents (Barnes & Bagenal, 1951).
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceBalanus crenatus is permanently attached to the substratum so would be removed upon substratum loss. The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily colonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994). Therefore recovery is predicted to be high. | High | High | Moderate | Moderate |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceBalanus crenatus can withstand covering by silt provided that the cirri can extend above the silt layer but smothering by 5cm of sediment would prevent feeding and could cause death. The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily colonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994). Therefore recovery is predicted to be high. | High | High | Moderate | Low |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceBalanus species are generally tolerant of moderate siltation but are intolerant of excessive siltation (Holt et al., 1995). Silt could clog the filter feeding apparatus imposing an energetic cost on clearing the cirri. The reduced growth rate of barnacles living on carapaces of Nephrops norvegicus compared to barnacles growing on rafts has been partly attributed to the increased levels of silt in the immediate vicinity of Nephrops norvegicus (Barnes & Bagenal, 1951). Therefore, Balanus crenatus is reported to have a low intolerance to siltation as growth only would be affected. The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily colonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994). Therefore recovery is predicted to be high. | Low | High | Low | Low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidenceBalanus crenatus has more permeable shell plates than other littoral barnacles and therefore loses water quicker and dies sooner when exposed to air. Foster (1971) recorded that Balanus crenatus adults of 6 mm and 11 mm diameter can withstand 17 hours and 40 hours of aerial exposure respectively. Similarly, Barnes et al. (1963) recorded that Balanus crenatus had a mean survival time of 14.4 hours in dry air. An increase in the period of desiccation would therefore lead to a depression in the upper limit of the species distribution. A decrease in the period of desiccation could lead to an extension of Balanus crenatus up the shore. The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily colonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994). Therefore recovery is predicted to be high. | High | High | Moderate | High |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceBalanus crenatus is vulnerable to desiccation upon aerial exposure. The shell plates are more permeable than other littoral barnacles, therefore it loses water and dies quicker. Foster (1971) recorded that adults of 6 mm and 11 mm diameter can withstand 17 hours and 40 hours of aerial exposure respectively. An increase in the period of emergence would lead to a depression in the upper limit of the species distribution. A decrease in the period of emersion could lead to an extension of Balanus crenatus up the shore. The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily colonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994). Therefore, recovery is predicted to be high. | High | High | Moderate | High |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceBalanus crenatus is found in a very wide range of water flow rates. However, Barnes & Bagenal (1951) found that the growth rate of Balanus crenatus epizoic on Nephrops norvegicus was considerably slower than animals on raft exposed panels. This was attributed to reduced currents and increased silt loading of water in the immediate vicinity of Nephrops norvegicus, so growth rate may be reduced if water flow rate decreases. On return to normal water flow rate the growth rate is predicted to rapidly recover. | Low | Very high | Very Low | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceBalanus crenatus is a boreal species, and is intolerant of increases in water temperature. In Queens Dock, Swansea where the water was on average 10 °C higher than average due to the effects of a condenser effluent, Balanus crenatus was replaced by the subtropical barnacle Balanus amphitrite. After the water temperature cooled Balanus crenatus returned (Naylor, 1965). It has a peak rate of cirral beating at 20 °C and all spontaneous activity ceases at about 25 °C (Southward, 1955). The species is more tolerant of lower temperatures. Balanus crenatus was unaffected during the severe winter of 1962-63, when average temperatures were 5 to 6 °C below normal (Crisp, 1964). The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily colonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994). Therefore recovery is predicted to be high. | High | High | Moderate | Moderate |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceAn increase in turbidity could be beneficial for Balanus crenatus, if the suspended particles are composed of organic matter. However, if the suspended particles are inedible, an energetic cost may be imposed on clearing the cirri. A reduction in light penetration could also reduce growth rate of phytoplankton and so limit zooplankton levels, which form the bulk of barnacles food. Barnes & Bagenal (1951) found that growth rate of Balanus crenatus epizoic on the mud-burrowing prawn Nephrops norvegicus was considerably slower than animals on raft exposed panels. This was attributed to reduced currents and increased silt loading of water in the immediate vicinity of Nephrops norvegicus. On return to normal turbidity levels the growth rate of Balanus crenatus would resume quickly. | Low | Very high | Very Low | Low |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceBalanus crenatus can tolerate all degrees of wave exposure. However, barnacle growth is greatest at exposed locations (Crisp, 1960), so a decrease in wave exposure may reduce growth rate of barnacles if no tidal stream is present, by reducing the renewal rate of the water and therefore the food supply. On return to normal wave exposure levels the growth rate would quickly resume. | Low | Very high | Very Low | Low |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidenceBarnacles are unlikely to be sensitive to noise. | Tolerant | Not relevant | Not sensitive | Low |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceBarnacles are unlikely to be sensitive to visual presence. | Tolerant | Not relevant | Not sensitive | Low |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceBalanus crenatus would probably be crushed by a heavy force, such as an anchor landing on it. However, it is small and individuals in fissures and crevices would probably survive. The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily colonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994) so recovery is predicted to be high. | Intermediate | High | Low | Low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceBalanus crenatus is permanently attached to the substratum and could not survive if it was removed. However, the species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily colonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994) so recovery is predicted to be high. | High | High | Moderate | Low |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceBarnacles have a low resilience to chemicals such as dispersants, dependant on the concentration and type of chemical involved (Holt et al., 1995). They are less intolerant than some species (e.g. Patella vulgata) to dispersants (Southward & Southward, 1978) and Balanus crenatus was the dominant species on pier pilings at a site subject to urban sewage pollution (Jakola & Gulliksen, 1987). Hoare & Hiscock (1974) found that Balanus crenatus survived near to an acidified halogenated effluent discharge where many other species were killed, suggesting a high tolerance to chemical contamination. Little information is available on the impact of endocrine disrupters on adult barnacles. Holt et al. (1995) concluded that barnacles are fairly sensitive to chemical pollution, therefore intolerance is reported as high. The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily recolonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994). Therefore, recovery is predicted to be high. | High | High | Moderate | Very low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceBarnacles accumulate heavy metals and store them as insoluble granules (Rainbow, 1987). Pyefinch & Mott (1948) recorded a median lethal concentration of 0.19 mg/l copper and 1.35 mg/l mercury, for Balanus crenatus over 24 hours. Barnacles may tolerate fairly high level of heavy metals in nature, for example they are found in Dulas Bay, Anglesey, where copper reaches concentrations of 24.5 µg/l, due to acid mine waste (Foster et al., 1978). The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily recolonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994). Therefore recovery is predicted to be high. | Intermediate | High | Low | Low |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceNo information is available on the intolerance of Balanus crenatus to hydrocarbons. However, other littoral barnacles generally have a high tolerance to oil (Holt et al., 1995) and were little impacted by the Torrey Canyon oil spill (Smith, 1968) so Balanus crenatus is probably fairly resistant to oil. The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily recolonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994). Therefore recovery is predicted to be high. | Low | High | Low | Very low |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceA slight increase in nutrient levels could be beneficial for barnacles by promoting growth of phytoplankton and therefore increasing food supplies. Indeed, Balanus crenatus was the dominant species on pier pilings, which were subject to urban pollution (Jakola & Gulliksen, 1987). However, a large increase in nutrients could cause barnacles to be killed by the dense overgrowth of ephemeral green algae (Holt et al., 1995). The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily recolonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994). Therefore recovery is predicted to be high. | Intermediate | High | Low | Very low |
Increase in salinity [Show more]Increase in salinity
EvidenceWhen subjected to sudden changes in salinity Balanus crenatus closes its opercular valves so that the blood is maintained temporarily at a constant osmotic concentration. Balanus crenatus can tolerate salinities down to 14 psu if given time to acclimate (Foster, 1970). At salinities below 6 psu motor activity ceases, respiration falls and the animal falls in to a "salt sleep". In this state the animals may survive in fresh water for 3 weeks, enabling them to withstand changes in salinity over moderately long periods (Barnes, 1953). | Low | Very high | Very Low | High |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceBalanus crenatus respires anaerobically so it can withstand some decrease in oxygen levels. When placed in wet nitrogen, where oxygen stress is maximal and desiccation stress is minimal, Balanus crenatus has a mean survival time of 3.2 days (Barnes et al., 1963). It is therefore predicted that the species would not survive low oxygen levels for a week, so intolerance is reported as high. The species is an important early colonizer of sublittoral rock surfaces (Kitching, 1937) and it heavily recolonized a site that was dredged for gravel within 7 months (Kenny & Rees, 1994). Therefore recovery is predicted to be high. | High | High | Moderate | Very low |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceNR | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceNR | Not relevant | Not relevant | Not relevant | Not relevant |
Additional information
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Balanus crenatus is an important initial colonizing species, perhaps obscuring material such as anti-fouling paint that would be toxic to other species. It is a source of food for Nucella lapillus in tidal sounds. Balanus crenatus is also grazed by Echinus esculentus and fish species probably nip its cirri. The plates of dead Balanus crenatus are probably an important part of the unique shell gravel banks in the Menai Strait, North Wales.Bibliography
Barnes, H. & Bagenal, T.B., 1951. Observations on Nephrops norvegicus and an epizoic population of Balanus crenatus. Journal of the Marine Biological Association of the United Kingdom, 30, 369-380.
Barnes, H. & Powell, H.T., 1953. The growth of Balanus balanoides and B. crenatus under varying conditions of submersion. Journal of the Marine Biological Association of the United Kingdom, 32, 107-127.
Barnes, H., 1953. The effect of lowered salinity on some barnacle nauplii. Journal of Animal Ecology, 22, 328-330.
Barnes, H., Finlayson, D.M. & Piatigorsky, J., 1963. The effect of desiccation and anaerobic conditions on the behaviour, survival and general metabolism of three common cirripedes. Journal of Animal Ecology, 32, 233-252.
Bassindale, R., 1964. British Barnacles. London: The Linnean Society of London.[Synopses of the British Fauna, no. 14.]
Clarke, G.L., 1947. Poisoning and recovery in barnacles and mussels. Biological Bulletin, Marine Biological Laboratory, Woods Hole, 92, 73-91.
Crisp, D.J. (ed.), 1964. The effects of the severe winter of 1962-63 on marine life in Britain. Journal of Animal Ecology, 33, 165-210.
Crisp, D.J., 1960. Factors influencing the growth rate of Balanus balanoides. Journal of Animal Ecology, 29, 95-110.
Donahue, W.H., Wang, R.T., Welch, M., & Nicol, J.A.C., 1977. Effects of water-soluble components of petroleum oils and aromatic hydrocarbons on barnacle larvae. Environmental Pollution, 13, 187-202.
Foster, B.A., 1970. Responses and acclimation to salinity in the adults of some balanomorph barnacles. Philosophical Transactions of the Royal Society of London, Series B, 256, 377-400.
Foster, P., Hunt, D.T.E. & Morris, A.W., 1978. Metals in an acid mine stream and estuary. Science of the Total Environment, 9, 75-86.
Hoare, R. & Hiscock, K., 1974. An ecological survey of the rocky coast adjacent to the effluent of a bromine extraction plant. Estuarine and Coastal Marine Science, 2 (4), 329-348.
Jakola, K.J. & Gulliksen, B., 1987. Benthic communities and their physical environment to urban pollution from the city of Tromso, Norway. Sarsia, 72, 173-182.
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Kendall, M.A., Bowman, R.S., Williamson, P. & Lewis, J.R., 1985. Annual variation in the recruitment of Semibalanus balanoides on the North Yorkshire coast 1969-1981. Journal of the Marine Biological Association of the United Kingdom, 65, 1009-1030.
Kenny, A.J. & Rees, H.L., 1994. The effects of marine gravel extraction on the macrobenthos: early post dredging recolonisation. Marine Pollution Bulletin, 28, 442-447.
Kitching, J.A., 1937. Studies in sublittoral ecology. II Recolonization at the upper margin of the sublittoral region; with a note on the denudation of Laminaria forest by storms. Journal of Ecology, 25, 482-495.
Mortlock, A.M., Fitzsimons, J.T.R. & Kerkaut, G.A., 1984. The effects of farnesol on the late stage nauplius and free swimming cypris larvae of Elminius modestus. Comparative Biochemistry and Physiology, 78A, 345-357.
Naylor, E., 1965. Effects of heated effluents upon marine and estuarine organisms. Advances in Marine Biology, 3, 63-103.
Pyefinch, K.A. & Mott, J.C., 1948. The sensitivity of barnacles and their larvae to copper and mercury. Journal of Experimental Biology, 25, 276-298.
Rainbow, P.S., 1984. An introduction to the biology of British littoral barnacles. Field Studies, 6, 1-51.
Rainbow, P.S., 1987. Heavy metals in barnacles. In Barnacle biology. Crustacean issues 5 (ed. A.J. Southward), 405-417. Rotterdam: A.A. Balkema.
Smith, J.E. (ed.), 1968. 'Torrey Canyon'. Pollution and marine life. Cambridge: Cambridge University Press.
Southward, A.J. & Southward, E.C., 1978. Recolonisation of rocky shores in Cornwall after use of toxic dispersants to clean up the Torrey Canyon spill. Journal of the Fisheries Research Board of Canada, 35, 682-706.
Southward, A.J., 1955. On the behaviour of barnacles. I. The relation of cirral and other activities to temperature. Journal of the Marine Biological Association of the United Kingdom, 34, 403-432.
Southward, A.J., 1998. New observations on barnacles (Crustacea: Cirripedia) of the Azores Region. Arquipelago, 16A, 11-27.
Tighe-Ford, D.J., 1977. Effects of juvenile hormone analogues on larval metamorphosis in the barnacle Elminius modestus Darwin. Journal of Experimental Marine Biology and Ecology, 26, 163-176.
Willemsen, P.R., Overbeke, K. & Suurmond, A., 1998. Repetitive testing of TBTO, Sea-nine 211 and farnesol using Balanus amphitrite (Darwin) cypris larvae: variability in larval sensitivity. Biofouling, 12, 133-147.
Wu, R.S.S., Lam, P.K.S. & Zhou, B.S., 1997. Effects of two oil dispersants on phototaxis and swimming behaviour of barnacle larvae. Hydrobiologia, 352, 9-16.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
Kent Wildlife Trust, 2018. Biological survey of the intertidal chalk reefs between Folkestone Warren and Kingsdown, Kent 2009-2011. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Lancashire Environment Record Network, 2018. LERN Records. Occurrence dataset: https://doi.org/10.15468/esxc9a accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2022. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset:https://doi.org/10.15468/aru16v accessed via GBIF.org on 2024-09-27.
Merseyside BioBank., 2018. Merseyside BioBank (unverified). Occurrence dataset: https://doi.org/10.15468/iou2ld accessed via GBIF.org on 2018-10-01.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-04
South East Wales Biodiversity Records Centre, 2018. SEWBReC Myriapods, Isopods, and allied species (South East Wales). Occurrence dataset: https://doi.org/10.15468/rvxsqs accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. Dr Mary Gillham Archive Project. Occurance dataset: http://www.sewbrec.org.uk/ accessed via NBNAtlas.org on 2018-10-02
Yorkshire Wildlife Trust, 2018. Yorkshire Wildlife Trust Shoresearch. Occurrence dataset: https://doi.org/10.15468/1nw3ch accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 17/05/2004