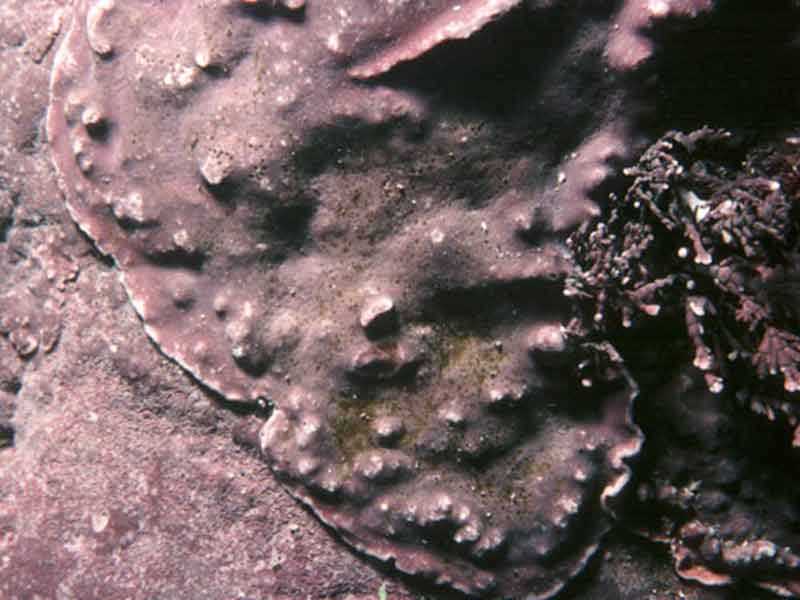
An encrusting coralline alga (Lithophyllum incrustans)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Dr Keith Hiscock | Refereed by | Dr Yvonne Chamberlain |
| Authority | Philippi, 1837 | ||
| Other common names | - | Synonyms | - |
Summary
Description
Calcified smooth pink or greyish pink crusts on rock, shells and holdfasts. Convoluted ridges present where neighbouring crusts meet. May become bleached when exposed to strong sunlight.
Recorded distribution in Britain and Ireland
Present all around the British Isles but rarer on the east coast between Yorkshire and east Kent. Encrusting coralline species are difficult to distinguish and few surveys record to species level. Its distribution is probably under recorded.Global distribution
Present in the Faroes, Norway at least south from Trondheimfjord to Spain and the Mediterranean. May also be present in Morocco and Mauritania. Recorded in South Africa (Chamberlain 1996)Habitat
Found on a wide range of hard rock substrata but may be unable to settle and grow well on soft rocks such as chalk, which is a major substratum type in the southeast of England. Present in rockpools and under algae in the littoral and usually covering rocks on the lower shore and sublittoral fringe. More rarely present in the sublittoral although only recorded in the sublittoral on the Sussex and Kent coast (Y. Chamberlain, pers. comm..).Depth range
Mid-littoral to at least 8m.Identifying features
- Crusts pale, greyish pink, thick and smooth but convoluted ridges often occur where adjacent crusts meet.
- Microscopic features include non-aligned thallus cells.
- Secondary growth extensive, often coaxial
- Margin thick.
- Tetra/bisporangial conceptacles with conspicuous calcified columella, pore canal of equal width throughout and not tapering.
- Old conceptacles are dumbbell-shaped and buried but can be seen if the thallus is snapped.
Additional information
Difficult to identify with certainty in the field and often recorded as 'lithothamnia' or 'encrusting Rhodophycota (indet.)' in surveys.
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Rhodophyta | Red seaweeds |
| Class | Florideophyceae | |
| Order | Corallinales | |
| Family | Lithophyllaceae | |
| Genus | Lithophyllum | |
| Authority | Philippi, 1837 | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | >30cm | ||
| Male size at maturity | |||
| Female size range | Medium-large(21-50cm) | ||
| Female size at maturity | |||
| Growth form | Crustose hard | ||
| Growth rate | <7mm/year | ||
| Body flexibility | None (less than 10 degrees) | ||
| Mobility | Sessile, permanent attachment | ||
| Characteristic feeding method | Autotroph | ||
| Diet/food source | Autotroph | ||
| Typically feeds on | Not relevant | ||
| Sociability | Colonial | ||
| Environmental position | Epilithic | ||
| Dependency | Independent. | ||
| Supports | None | ||
| Is the species harmful? | No | ||
Biology information
Dominant in rockpools and over much of the lower shore and sublittoral fringe at least. Covers the surface of rocks under canopies of algae.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast, Offshore seabed, Strait or Sound, Sea loch or Sea lough, Ria or Voe |
| Biological zone preferences | Lower eulittoral, Mid eulittoral, Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Rockpools |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Very strong > 6 knots (>3 m/sec.), Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Exposed, Extremely exposed, Moderately exposed, Sheltered, Very exposed, Very sheltered |
| Salinity preferences | Full (30-40 psu), Variable (18-40 psu) |
| Depth range | Mid-littoral to at least 8m. |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
No text enteredLife history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | >1,000,000 |
| Generation time | Insufficient information |
| Age at maturity | Insufficient information |
| Season | October - April |
| Life span | 20-100 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Spores (sexual / asexual) |
| Duration of larval stage | No information |
| Larval dispersal potential | Greater than 10 km |
| Larval settlement period | Insufficient information |
Life history information
Gametangial and tetrasporangial plants occur commonly on some shores in Devon and Cornwall but are rare in the north. The 'Time of first and last gamete' refers to the time when reproductive types occur however, some conceptacles are present throughout the year. (Irvine & Chamberlain 1994.) Assuming one layer of conceptacles is produced each year, plants up to 30 years old are reported (Edyvean pers. comm.. in Irvine & Chamberlain 1994). Reproductive types occur from October to April but tail-off into summer. It has been calculated that 1 mm x 1mm of reproductive thallus produces 17.5 million bispores per year with average settlement of only 55 sporelings/year (Edyvean & Ford 1984)Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceLithophyllum incrustans is permanently attached to the substratum. Therefore, loss of substratum will entail loss of this species. Spores will settle and new colonies will arise rapidly on bare substratum but growth rate is slow (2-7 mm per annum - see Irvine & Chamberlain 1994). Colonies may be up to 30 years old (Edyvean in Irvine & Chamberlain 1994). | High | Low | High | High |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceEncrusting coralline algae are frequently subject to cover by sediment and appear to survive well. | Low | Very high | Very Low | Moderate |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceSilt settling onto encrusting coralline algae may be removed by production of mucus. Reduction in light penetration may reduce or prevent photosynthesis but, in the situation where the increased siltation is for a short period, colonies are likely to survive. If death occurred, recoverability would be low (see additional information). | Low | Very high | Very Low | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceEncrusting coralline algae are likely to benefit from a decrease in siltation. | Tolerant* | Not relevant | Not sensitive* | High |
Desiccation [Show more]Desiccation
EvidenceOccurrence of encrusting coralline algae seems to be critically determined by exposure to air and sunlight. Colonies survive in damp conditions under algal canopies or in pools but not on open rock where desiccation effects are important. Harkins & Hartnoll (1985) noted that the presence of fucoid canopies allowed encrusting corallines to extend their upper limit higher on the shore. Canopy removal experiments in the Isle of Man, noted that encrusting corallines died within a week of removal of the protection canopy of Fucus serratus (Hawkins & Harkin, 1985). Removal of the Laminaria digitata canopy lower on the shore resulted in bleaching of encrusting corallines (Hawkins & Harkin, 1985) probably due to increased light intensity (see turbidity). Hawkins & Hartnoll (1985) reported extensive damage to encrusting and articulate corallines during the hot summer of 1983 at several sites in Britain. Therefore, desiccation is an important factor limiting the distribution of encrusting coralline algae on the shore, and an intolerance of high has been recorded. Recovery is likely to be slow (see additional information, below). | High | Low | High | High |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceOccurrence of encrusting calcareous algae seems to be critically determined by exposure to air and sunlight. Colonies survive in damp conditions under algae or in pools but not on open rock where desiccation effects are important. Increased emergence will increase the risk of desiccation (see above). If killed recovery will be slow (see additional information below). | High | Low | High | High |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceThere may be less light reaching the seabed for photosynthesis but it is not expected that established colonies of Lithophyllum incrustans will be adversely affected. | Tolerant | Not relevant | Not sensitive | Moderate |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceColonies of Lithophyllum incrustans appear to thrive especially in conditions exposed to strong water movement, including very strong wave action. Increase in the strength of tidal flow over colonies in therefore unlikely to have an adverse impact and may remove silt so that there will be a favourable effect. | Low | Very high | Very Low | Moderate |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceLithophyllum incrustans tolerates a wide range of water flow conditions. However, where wave action is not the primary source of water movement, a marked decrease in water flow may have an adverse effect especially if it allows siltation to occur. In the situation where increased siltation is for a short period, colonies are likely to survive. However, if water flow is reduced over a long period or permanently, there may be mortality and loss. | Low | Very high | Very Low | Moderate |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceLithophyllum incrustans occurs in a wide geographical range in temperatures that are much warmer (air and water) than in Britain and Ireland. It is therefore, probalby tolerant of an increase in temperature. However, increased temperature may result in an increased risk of desiccation (see above). | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceLithophyllum incrustans occurs in a wide geographical range in temperatures that are much colder (air and water) than in Britain and Ireland. It is therefore likely to tolerate a decrease in temperature, at the benchmark level. | Tolerant | Not relevant | Not sensitive | Moderate |
Increase in turbidity [Show more]Increase in turbidity
EvidenceReduction in light penetration may reduce or prevent photosynthesis but, colonies are likely to survive. However, at the lower limit of its range, colonies will most likely be adversely affected by long-term (< one year) change. Removal of the protective canopy of Laminaria digitata in the Isle of Man (Hawkins & Harkin, 1985) resulted in bleaching of encrusting corallines, suggesting that Lithophyllum incrustans may be intolerant of high light intensities. As a shade tolerant species, increased light due to decreased turbidity in the absence of shading algae may have adverse affects. | Low | Very high | Very Low | Low |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceThe major effect is likely to be increased light penetration which will have a favourable effect on colonies of Lithophyllum incrustans. | Tolerant* | Not relevant | Not sensitive* | Moderate |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceColonies of Lithophyllum incrustans appear to thrive in conditions exposed to strong water movement. Irvine & Chamberlain (1994) observe that the species is best developed on wave exposed shores. In some situations where water movement has been low, increased exposure to wave action may be beneficial but in many situations, an assessment of 'tolerant' is appropriate. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceA marked decrease in wave exposure may have an adverse effect on growth especially if it allows siltation to occur. However, mortality would only be expected if the decrease in wave exposure was for a long period. Therefore intolerance is assessed as low. | Low | Immediate | Not sensitive | Moderate |
Noise [Show more]Noise
EvidenceLithophyllum incrustans has no known sound receptors. | Tolerant | Not relevant | Not sensitive | High |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceLithophyllum incrustans has no known visual receptors. | Tolerant | Not relevant | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceLittler & Kauker (1984) suggested that crustose algal forms were resistant to predation, sand scour and wave shear. Colonies on rock may be completely removed over part of the area affected but recolonize from parts protected in crevices or unaffected parts. Remaining parts of the crust will expand once the source of abrasion is removed.
Spores will settle and new colonies will arise rapidly on bare substratum but growth rate is slow (2-7 mm per annum - see Irvine & Chamberlain 1994). Colonies are up to 30 years old (Edyvean in Irvine & Chamberlain 1994) | Intermediate | High | Low | Moderate |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceRemoval from the substratum for such an encrusting species is unlikely and it is more likely that the substratum (e.g. cobbles or boulders) with the organism attached will be moved. Providing that the move is to a similar habitat, the effect is likely to be minimal. | Low | Very high | Very Low | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceLittle information has been found. Hoare & Hiscock (1974) recorded that 'lithothamnia' was absent from the rocky shore up to 150 m distant from an acidified halogenated effluent. Once the impact is removed, spores will settle and new colonies will arise rapidly on bare substratum but growth rate is slow (see additional information below). | High | Low | High | Low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceWhere exposed to direct contact with fresh hydrocarbons, encrusting coralline algae appear to have a high intolerance. Crump et al. (1999) describe "dramatic and extensive bleaching" of 'Lithothamnia' following the Sea Empress oil spill. Observations following the Don Marika oil spill (K. Hiscock, own observations) were of rockpools with completely bleached coralline algae. However, Chamberlain (1996) observed that although Lithophyllum incrustans was quickly affected by oil during the Sea Empress spill, recovery occurred within about a year. The oil was found to have destroyed about one third of the thallus thickness but regeneration occurred from thallus filaments below the damaged area. A recoverability of high is therefore suggested. If colonies were completely destroyed new growth would be slow and, because of low growth rates, recoverability would be low (see additional information below). | High | High | Moderate | Moderate |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceSewage pollution (as a source of nutrients) appears to have little or no effect. In the case of erect coralline algae, numbers might increase (reviewed in Fletcher 1996). Increased nutrients may result in overgrowth by other algae. Where mortality occurs, spores will settle and new colonies will arise rapidly on bare substratum but growth rate is slow (see additional information below). | Low | High | Low | Low |
Increase in salinity [Show more]Increase in salinity
EvidenceLithophyllum incrustans lives in full salinity seawater. Increase in salinity may occur if evaporation in intertidal pools occurred. However, no information has been found on tolerance to hypersaline conditions. | No information | Not relevant | No information | Not relevant |
Decrease in salinity [Show more]Decrease in salinity
EvidenceLittle direct information on the effect of salinity change on encrusting coralline algae was found but red seaweeds are generally more intolerant of reduced salinity conditions than brown or green algae (Kain & Norton 1990). However, in the case of short-term change, encrusting coralline algae must be able to withstand the effects of heavy rain in diluting seawater in pools and in run-off as entirely freshwater over exposed corallines. Recovery is likely to be fairly rapid if, as in the impact of oil spills (see above), only the cell layers near the surface are adversely affected. If colonies were completely destroyed new growth would be slow and, because of low growth rates, recoverability would be low (see additional information below). | Intermediate | High | Low | Low |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceNo information concerning the effects of oxygen levels on encrusting corallines were found. | No information | Not relevant | No information | Not relevant |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceCurrently, there appear to be no non-native species in Britain that adversely affect encrusting coralline algae. However, aggressive invasive species could out-compete Lithophyllum incrustans and over-grow it. | No information | Not relevant | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceIt is not believed that this species would be extracted. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceExtraction of species such as kelps, where encrusting coralline algae grow on holdfasts, may have a small localised adverse effect but growth from surrounding crusts would fill any gaps in cover and re-growth of encrusting corallines occurs on re-growth of kelps. | Intermediate | High | Low | Moderate |
Additional information
Recoverability. If death occurred, recoverability will be slow. Spores will settle and new colonies will arise rapidly on bare substratum but the growth rate is slow (2-7 mm per annum - see Irvine & Chamberlain 1994). Colonies are up to 30 years old (Edyvean pers. comm., in Irvine & Chamberlain 1994).
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | Not relevant |
Importance information
Lithophyllum incrustans is a key structuring species that dominates extensive rocky areas to the exclusion of other encrusting species.Bibliography
Chamberlain, Y.M., 1996. Lithophylloid Corallinaceae (Rhodophycota) of the genera Lithophyllum and Titausderma from southern Africa. Phycologia, 35, 204-221.
Chamberlain, Y.M., 1997. Investigation of the condition of crustose coralline red algae in Pembrokeshire after the Sea Empress disaster 15-21 February 1996. Countryside Council for Wales Sea Empress Report no. 178, 40 pp.
Crump, R.G., Morley, H.S., & Williams, A.D., 1999. West Angle Bay, a case study. Littoral monitoring of permanent quadrats before and after the Sea Empress oil spill. Field Studies, 9, 497-511.
Edyvean, R.G.J. & Ford, H., 1984b. Population biology of the crustose red alga Lithophyllum incrustans Phil. 3. The effects of local environmental variables. Biological Journal of the Linnean Society, 23, 365-374.
Hardy, F.G. & Guiry, M.D., 2003. A check-list and atlas of the seaweeds of Britain and Ireland. London: British Phycological Society
Hawkins, S.J. & Harkin, E., 1985. Preliminary canopy removal experiments in algal dominated communities low on the shore and in the shallow subtidal on the Isle of Man. Botanica Marina, 28, 223-30.
Hawkins, S.J. & Hartnoll, R.G., 1985. Factors determining the upper limits of intertidal canopy-forming algae. Marine Ecology Progress Series, 20, 265-271.
Hiscock, S., 1986b. A field key to the British Red Seaweeds. Taunton: Field Studies Council. [Occasional Publication No.13]
Hoare, R. & Hiscock, K., 1974. An ecological survey of the rocky coast adjacent to the effluent of a bromine extraction plant. Estuarine and Coastal Marine Science, 2 (4), 329-348.
Irvine, L. M. & Chamberlain, Y. M., 1994. Seaweeds of the British Isles, vol. 1. Rhodophyta, Part 2B Corallinales, Hildenbrandiales. London: Her Majesty's Stationery Office.
Kain, J.M., & Norton, T.A., 1990. Marine Ecology. In Biology of the Red Algae, (ed. K.M. Cole & Sheath, R.G.). Cambridge: Cambridge University Press.
Littler, M.M., & Kauker, B.J., 1984. Heterotrichy and survival strategies in the red alga Corallina officinalis L. Botanica Marina, 27, 37-44.
Littler, M.W., 1972. The Crustose Corallinaceae. Oceanography and Marine Biology: an Annual Review, 10, 311-347.
Schiel, D.R. & Taylor, D.I., 1999. Effects of trampling on a rocky intertidal algal assemblage in southern New Zealand. Journal of Experimental Marine Biology and Ecology, 235, 213-235.
Datasets
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2014. Occurrence dataset: https://doi.org/10.15468/erweal accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2016. Occurrence dataset: https://doi.org/10.15468/146yiz accessed via GBIF.org on 2018-09-27.
Manx Biological Recording Partnership, 2017. Isle of Man wildlife records from 01/01/2000 to 13/02/2017. Occurrence dataset: https://doi.org/10.15468/mopwow accessed via GBIF.org on 2018-10-01.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-02
Outer Hebrides Biological Recording, 2018. Non-vascular Plants, Outer Hebrides. Occurrence dataset: https://doi.org/10.15468/goidos accessed via GBIF.org on 2018-10-01.
Royal Botanic Garden Edinburgh, 2018. Royal Botanic Garden Edinburgh Herbarium (E). Occurrence dataset: https://doi.org/10.15468/ypoair accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 01/07/2003