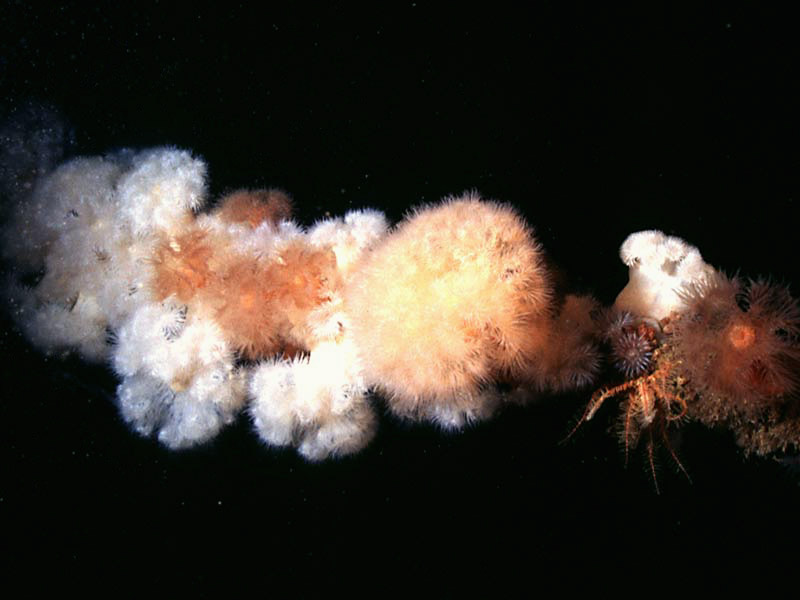
Plumose anemone (Metridium senile)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Dr Keith Hiscock & Emily Wilson | Refereed by | This information is not refereed |
| Authority | (Linnaeus, 1761) | ||
| Other common names | - | Synonyms | Metridium senile (Linnaeus, 1761), Metridium dianthus (Ellis, 1768) |
Summary
Description
Metridium senile is an anemone of very variable form. The base is wider than the column and often irregular. When expanded, the numerous tentacles form a 'plume' above a conspicuous parapet at the top of the smooth column. Large individuals may be 30 cm high. The colour is plain, commonly white orange or dark green but brown, grey or occasionally red or yellow varieties occur.
Recorded distribution in Britain and Ireland
All British and Irish coasts.Global distribution
See additional information below.Habitat
Attached to any suitable hard substratum in overhangs, caves and beneath boulders on the lower shore, and on pier piles and rock faces to at least 100 m.Depth range
Lower shore to considerable depths.Identifying features
- The base is wider than column, moderately or firmly adherent, outline often ragged due to basal laceration.
- The column is divided into a smooth scapus and relatively long capitulum, with a parapet and fosse; in full expansion, the parapet often forms a salient, collar-like ring.
- The disc is fairly wide and there are prominent protruding lips around the mouth.
- The form 'dianthus' is tall (up to 30 cm), up to 15 cm across the base and has several thousand short slender tentacles which give the expanded anemone a fluffy appearance.
- The form 'pallidus' (pallidum in Bucklin, 1985) is small, not generally exceeding 2.5 cm across the base) and the tentacles are long and slender, not usually exceeding 200 in number.
Additional information
Fautin (2016b) recognised both Metridium senile and M. dianthus as valid synonyms but noted that the use of the names has varied over time. Manuel (1988) describes two distinctive varieties of Metridium senile var. dianthus is large with a tall column when expanded. The disc is deeply waved or folded. The many tentacles give a 'fluffy' appearance. Individuals may be 30 cm in height, with a basal diameter and tentacle span of 15 cm or more. Var. pallidus is a small form not exceeding 2.5 cm across the base with a flat disc without folds. Bucklin (1985) investigated biochemical genetic variation and concluded the presence of two morphs of Metridium senile but that they were variants resulting from different environmental conditions and were not taxonomically distinct and, therefore, not 'varieties' as described in many texts.
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Cnidaria | Sea anemones, corals, sea firs & jellyfish |
| Class | Anthozoa | Sea anemones, soft & cup corals, sea pens & sea pansies |
| Order | Actiniaria | |
| Family | Metridiidae | |
| Genus | Metridium | |
| Authority | (Linnaeus, 1761) | |
| Recent Synonyms | Metridium senile (Linnaeus, 1761)Metridium dianthus (Ellis, 1768) | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | 30cm | ||
| Male size at maturity | |||
| Female size range | Medium-large(21-50cm) | ||
| Female size at maturity | |||
| Growth form | Radial | ||
| Growth rate | 9cm/month | ||
| Body flexibility | High (greater than 45 degrees) | ||
| Mobility | Sedentary, temporary attachment | ||
| Characteristic feeding method | Passive suspension feeder | ||
| Diet/food source | Omnivore | ||
| Typically feeds on | Zooplankton but also larger prey. (See additional information.) | ||
| Sociability | |||
| Environmental position | Epilithic | ||
| Dependency | Independent. | ||
| Supports | Substratum Aeolidia papillosa, Pycnogonum littorale. | ||
| Is the species harmful? | No Toxicity is equivocal. Metridium senile is eaten by some fish (for instance black bream Spondyliosoma cantharus (Mattacola, 1976)) and therefore appears low toxicity. However, nematocysts are present and some stinging is possible in sensitive humans. | ||
Biology information
Growth rate. Bucklin (1987a) observed that Metridium senile grew rapidly in laboratory culture when fed daily reaching a mean pedal diameter of 45 cm after five months.
Feeding. Anthony (1997) noted that small anemones had the highest feeding efficiency at moderate to high flow regimes (which might help to account for the prevalence of small individuals at wave-exposed locations). Robbins & Schick (1980) found that current strength was the principal cause of expansion in Metridium senile rather than food availability. The greatest percentage of the anemones were expanded when the tide was running than at slack water. Examination of waste pellets of Metridium senile on wharf pilings in Monterey Bay, California (Purcell, 1976) revealed a diet of copepods, polychaete larvae, bivalve and gastropod veligers, copepod nauplii, and barnacle nauplii and cyprids. Sebens (1984) demonstrated that barnacle cyprids, ascidian larvae and gammarid amphipods were the preferred food of Metridium senile over invertebrate eggs, foraminiferans, calanoid and harpacticoid copepods and ostracods.
Predation. Metridium senile is subject to predation from both small and large consumers. The life stages of the sea spider Pycnogonum littorale found feeding on the anemone were reported by Wilhelm et al. (1997). The sea slug Aeolidia papillosa also feeds on Metridium senile (see, for instance, Reidy, 1996; Sebens, 1985). Sebens (1985) reported heavy mortality every winter in the Gulf of Maine, USA from Aeolidia papillosa. However, infestations may be sporadic. Gorzula & Cameron (1976) reported a population boom of Aeolidia papillosa at Millport, Firth of Clyde during February 1974 and that it was the third recorded that century. Effects on the Metridium senile population were considerable although the slugs vanished after four weeks. Epitonid snails (wentletraps) feed on anemones and Perron (1978) observed that Metridium senile was the preferred prey of Epitonium greenlandicum in the Bay of Fundy. Whether north-east Atlantic wentletraps feed on Metridium senile is uncertain although Graham (1988) notes that Epitonium clathrus feeds on Anemonia sulcata. Larger species that eat whole anemones include the black bream Spondyliosoma cantharus (Mattacola, 1976) and, in New Foundland, the winter flounder Pseudopleuronectes americanus (Keats, 1990).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Offshore seabed, Ria or Voe, Strait or Sound |
| Biological zone preferences | Lower circalittoral, Lower infralittoral, Sublittoral fringe, Upper circalittoral, Upper infralittoral |
| Substratum / habitat preferences | Artificial (man-made), Bedrock, Biogenic reef, Caves, Large to very large boulders, Overhangs |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Very strong > 6 knots (>3 m/sec.) |
| Wave exposure preferences | Exposed, Extremely exposed, Extremely sheltered, Moderately exposed, Sheltered, Very exposed, Very sheltered |
| Salinity preferences | Full (30-40 psu) |
| Depth range | Lower shore to considerable depths. |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
The species occurs from Biscay to Scandinavia in the northeast Atlantic. It is unknown from the western basin of the Mediterranean but recorded from the Adriatic (where it is believed to have been introduced) (Manual 1988). The same species occurs on the west and east coasts of North America. It has recently (Griffiths et al., 1996) been reported from Table Bay Harbour in South Africa where it was probably introduced from Europe. Both dianthus and pallidum forms may occur in estuaries and pallidum in brackish creeks. Braber & Borghouts (1977) recorded Metridium senile from salinities as low as 10 ppt Chlorinity (about 19 psu) in the Delta Region of the Netherlands.Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | No information |
| Fecundity (number of eggs) | No information |
| Generation time | Insufficient information |
| Age at maturity | Insufficient information |
| Season | August - September |
| Life span | See additional information |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Lecithotrophic |
| Duration of larval stage | 1-6 months |
| Larval dispersal potential | Greater than 10 km |
| Larval settlement period | Insufficient information |
Life history information
The Plymouth Marine Fauna (Marine Biological Association, 1957) reports that ova and sperm are produced in August and September at Plymouth. Bull (1939b) records that ova and sperm are given off at intervals throughout the year in north-east England. An account of reproductive cycles in Californian Metridium senile, where spawning occurred in September and October, is given in Bucklin (1982). Sebens (1985) suggests that the larva is lecithotrophic but has a 'pre-metamorphosis' period of months, a dispersal potential of >10,000m and a colonization rate of 5-10 years. Metridium senile colonizes areas aggressively. In studies of succession in rock wall communities in the Gulf of Maine, USA, Sebens (1985), the anemone was a late colonizer but grew over earlier colonizers and used specialized 'catch-tentacles' to damage other anemones and soft corals. The presence of such 'catch-tentacles' is also reported for Metridium senile in Britain (Williamson, 1975).
Growth is rapid. Bucklin (1985), working in Britain, found that for Metridium senile f. dianthus fragments and for f. pallidum newly settled individuals, a growth rate of up to 0.6 mm and 0.8 mm in pedal diameter per day occurred respectively. Bucklin (1987a) found that, for Metridium senile from California, individuals showed rapid growth to large sizes when fed at frequent intervals. Mean size grew steadily during the first eight months then levelled off. An increase from 5 cm² pedal disk area to 45 cm² occurred within 12 months. No information on longevity has been found although it would be expected that individuals are long-lived (10 years+).
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceAdult Metridium senile live attached to the substratum and will be removed with it. Re-colonization is likely to be rapid and occur within two or three years, although full recovery of dominance may take in excess of five years (see additional information below). Larvae of Metridium dianthus settle readily from the plankton and are known to colonize new structures such as wrecks and jetty piles within two to three years (K. Hiscock, own observations). The growth rate is rapid and a large size is reached in well-fed individuals within about nine months (see Bucklin, 1985). It is also possible that migration might occur from nearby adult populations as is believed to occur in re-colonizing previously de-oxygenated areas (Wahl, 1985). Therefore an intolerance of high and a recoverability of high has been recorded. | High | High | Moderate | High |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceSmothering by sediment is unlikely to occur as the anemone can expand above a layer of silt. Smothering by more impervious material is likely to result in anoxia and Metridium senile is very tolerant of deoxygenated conditions (Wahl, 1884). However, smothering may result in the anemone withdrawing its tentacles and shrinking or expending energy to rise above the sediment so that an intolerance of low is appropriate. | Low | Immediate | Not sensitive | High |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceMetridium senile can produce mucus to clear itself of silt. The production of mucus may have an energetic consequence and an intolerance of low is recorded. | Low | Immediate | Not sensitive | High |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceMetridium senile does not rely on silt for feeding and may benefit by not having to produce mucus to slough off silt. However, a decrease in siltation may result in decreased turbidity but see 'turbidity' for effects. | Tolerant* | Not relevant | Not sensitive* | Moderate |
Desiccation [Show more]Desiccation
EvidenceMetridium senile does not generally occur in the intertidal although may be present under piers and under overhangs, i.e. in shaded locations. They can produce mucus which would provide some protection from desiccation but, on the open shore, are most likely vulnerable and would be adversely affected. At least some may be killed following a one-hour exposure to sunshine whilst, in the case of a longer-term alteration in tidal level (see increase in emergence) migration is likely. An intolerance of intermediate is suggested but with a low confidence. For recoverability, see additional information below. | Intermediate | High | Low | Low |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceMetridium senile does not generally occur in the intertidal but may be present under piers and under overhangs, i.e. in shaded locations. In the case of an increase in emergence, migration is likely. Metridium senile is capable of detaching and floating away when conditions become undesirable. For instance, Wahl (1984, 1985) noted that in anoxic conditions, anemones detached from the substratum and drifted away. Although individual anemones are therefore unlikely to be killed by an increase in emergence, they will be lost from a particular location and therefore intolerance is described as high. For recoverability, see additional information below. | High | High | Moderate | High |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceMetridium senile is predominantly a subtidal species and therefore decrease in emergence would provide new habitats for it to settle in. | Tolerant* | Not relevant | Not sensitive* | High |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceMetridium senile is a passive suspension feeder relying on water currents to bring food. Hiscock (1983) describes the reaction of Metridium dianthus to increasing flow rate (to 90 cm/s) in a flume. The anemones were stimulated to expand tentacles as flow increased and only withdrew them at flow rates in excess of 70 cm/s. They were not swept away. Whilst large Metridium senile thrive in tidal narrows where surface velocity may be in the region of 3-5 knots, they do not appear to occur in very strong tidal flows (exceeding 5 knots) such as in the Gulf of Corryvreckan or Strangford Lough Narrows. An increase in water flow rate to about 5 knots is therefore likely to favour settlement and growth of Metridium senile especially because of increased food supply whilst above 5 knots, adverse effects including the inability to feed and possible displacement occur. As the benchmark is for an increase from moderately strong to very strong (>6 knots) water flow, adverse effects may occur and an intolerance assessment of intermediate is suggested to reflect the possible loss of some individuals. | Intermediate | Very high | Low | Moderate |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceMetridium senile is a passive suspension feeder relying on water currents to bring food. A decrease in water flow rate is likely to significantly reduce opportunities for feeding and the growth rate and expansion of populations by basal laceration may be reduced. Established individuals are likely to survive for the period of decreased flow prescribed in the benchmark and therefore an intolerance of low is suggested. However, in the long-term, the population is likely to decline through predation and detachment and not be replaced so that an intolerance, in that situation, of intermediate or high would be appropriate. For recoverability, see additional information. | Low | Very high | Very Low | Moderate |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceMetridium senile occurs in the Bay of Biscay south of Britain but also in the Adriatic (Manual, 1988) suggesting that it would be tolerant of long-term increases in temperature in Britain and Ireland. No evidence has been found of adverse effects of short-term temperature increase on anemones occurring, for instance, adjacent to thermal effluents. Therefore an assessment of tolerant is made but with low confidence. | Tolerant | Not relevant | Not sensitive | Low |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceMetridium senile occurs in much colder waters than those surrounding Britain and Ireland. Crisp (1964) records (but from only one location) that the anemone was unaffected by the cold winter of 1962-63. | Tolerant | Not relevant | Not sensitive | High |
Increase in turbidity [Show more]Increase in turbidity
EvidenceMetridium senile may benefit from increase in turbidity as algal growth on hard substrata will be reduced. For instance, Svane & Groendahl (1988) found that, in comparison with records from 1926-29, Metridium senile had colonized areas in the Gullmar Fjord where it had not been recorded and ascribed the reason to a possible increase in turbidity (and tolerance of pollution). | Tolerant* | Not relevant | Not sensitive* | Moderate |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceMetridium senile may be adversely affected by a decrease in turbidity as algal growth on hard substrata will be increased. Svane & Groendahl (1988) found that, in comparison with records from 1926-29, Metridium senile had colonized areas in the Gullmar Fjord where it had not been recorded and ascribed the reason to a possible increase in turbidity (and tolerance of pollution). However, evidence for an adverse effect on Metridium senile due to a decrease in turbidity is poor and, although an intolerance of intermediate is assigned, confidence is very low. For recoverability, see additional information below. | Intermediate | High | Low | |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceMetridium senile occurs in greatest abundance in the most wave sheltered situations (but usually with significant tidal flow) and in the most extremely wave exposed conditions where populations are of small individuals in shallow surge situations. The likely impact of increased wave exposure on the large individuals that typically occur in wave sheltered situations is especially considered here. Those wave sheltered situations may, from time to time, be subject to strong wave action when winds blow from a direction that is not prevailing. For instance, on the open east coast of Lundy, Metridium senile occurs on shallow jetty piles and on a wreck at 15 m depth where they persist despite occasional strong wave action during easterly winds. It seems most likely that individuals close and shrink down during strong wave action but survive. Metridium senile is strongly adherent and when closed probably produces little resistance to water flow. Therefore, the impact will be of decreased feeding opportunities and perhaps loss of condition but recovery will be rapid. For situations where wave exposure increases in already wave exposed situations, it might be that abundance of Metridium senile will increase. Bucklin (1987b) found that the small size of intertidal populations was imposed, most likely, by limited food and feeding time and damage from wave action, which stimulates fragmentation. Their reaction to strong wave action seems to be to increase in numbers but remain small. | Low | Immediate | Not sensitive | Low |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceMetridium senile occurs in greatest abundance in the most wave sheltered (but usually with significant tidal flow) situations. Therefore, a decrease in wave exposure may favour colonization by the anemone. However, decreased wave exposure at the sort of extremely exposed locations where populations of small individuals occur may adversely affect the survival of those populations. For the purpose of this review, the most widespread occurrence of Metridium senile (in sheltered locations) is applied and where a decrease in wave exposure might be favourable. | Tolerant* | Not relevant | Not sensitive* | Moderate |
Noise [Show more]Noise
EvidenceMetridium senile is likely to have poor ability for detection of noise vibrations and as such is unlikely to be sensitive to noise. | Tolerant | Not relevant | Not sensitive | High |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceMetridium senile has very limited, if any, ability for visual perception. The anemone is unlikely to be sensitive to visual presence. | Tolerant | Not relevant | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceThe anemone is soft, flexible and can reform its attachment to the substratum. Physical impact is likely to cause damage and mortality to exposed individuals but, because the species habitually reproduces by basal laceration, it seems likely that torn individuals will re-grow. Although some individuals will be displaced or killed, at the level of the benchmark, effects will be intermediate and recovery likely to be very high. In more extensive events of physical disturbance, intolerance is likely to be more similar to substratum removal. | Intermediate | Very high | Low | High |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceWahl (1984) observed that anemones detached from the substratum during the first week of deoxygenation in the Inner Flensburg Fjord and may drift away eventually to resettle. Metridium senile therefore seems able to survive displacement from the substratum but, presumably, may be damaged during the displacement in which case some repair may be needed. | Low | Very high | Very Low | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceMercier et al. (1998) exposed Metridium senile to tributyltin contamination in surrounding water and in contaminated food. The species produced mucus 48 hours after exposure to contaminated seawater. TBT was metabolized but accumulated lower levels of butyltins leading the authors to suggest that they seemed vulnerable to TBT contamination. However, Mercier et al. (1998) do not indicate any mortality and, since Metridium senile is a major component of jetty pile communities immediately adjacent to large vessels coated with TBT antifouling paints, intolerance is assessed as low specifically to TBT. | Low | Very high | Very Low | Moderate |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceNo information has been found of accumulation or effects of heavy metals on Metridium senile. | No information | Not relevant | No information | Not relevant |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceMetridium senile is a major component of jetty pile communities immediately adjacent to areas subject, in previous times, to the discharge of oily ballast and also, in Milford Haven, to a refinery effluent containing hydrocarbons (K. Hiscock, own observations). The anemone is able to produce mucus as a protective mechanism should oil settle onto individuals. No records have been found of any mortality of Metridium senile during oil spills or of any experimental studies of effects. Therefore, although an intolerance of low is indicated, it is with low confidence. | Low | Immediate | Not sensitive | Low |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceNo information has been found. | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceMetridium senile may benefit from an increase in nutrients. For instance, Svane & Groendahl (1988) found that, in comparison with records from 1926-29, Metridium senile had colonized areas in the Gullmar Fjord where it had not been recorded and ascribed the reason to possible tolerance of pollution from a pulp mill (and increase in turbidity). Therefore tolerant* is indicated. | Tolerant* | Not relevant | Not sensitive* | Very low |
Increase in salinity [Show more]Increase in salinity
EvidenceThe species occurs in full salinity but seems to thrive in variable salinity conditions (for instance in tidal narrows at the entrance to estuaries and on jetty piles in enclosed areas). It might be that higher salinity conditions would reduce its competitiveness and an intolerance of 'low' is suggested. | Low | Immediate | Not sensitive | Low |
Decrease in salinity [Show more]Decrease in salinity
EvidenceAlthough Metridium senile is predominantly marine, the species does penetrate into estuaries. Braber & Borghouts (1977) found that Metridium senile occurred in about 10 ppt Chlorinity (about 19 psu) in the Delta Region of the Netherlands suggesting that it would be tolerant of reduced salinity conditions. Shumway (1978) found that, during exposure to 50% seawater, animals retracted their tentacles whilst animals exposed to fluctuating salinity, contracted their body wall and produced copious mucus. Therefore, the species seems to have a high tolerance to reduction in salinity but may have to retract tentacles, suffer reduced opportunity to feed and expend energy to produce mucus. Intolerance has therefore been assessed as low suggesting that individuals are unlikely to be killed by changes at the level of the benchmark. Recovery is in terms of condition and is therefore very high. | Low | Very high | Very Low | High |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceWahl (1984, 1985) noted that the LC50 value for Metridium senile in anoxic conditions is about three weeks and that none survive beyond six weeks. He observed that anemones detached from the substratum during the first week of deoxygenation in the Inner Flensburg Fjord and may drift away. When oxygen is lacking, Metridium senile diminishes the body surface area. At the level of the benchmark, Metridium senile is not sensitive and even in extreme conditions seems able to survive for some time and then detach. Although, at the benchmark level, recoverability is 'Not Relevant', it seems that re-colonization can be very rapid from nearby individuals. However, following a severe effect, it might take several years for re-colonization to the previous cover to occur (see additional information below). | Tolerant | Not relevant | Not sensitive | High |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceNo information has been found of effects of any microbial pathogens. | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceNo non-native species currently present in Britain and Ireland are known to have any impact on populations of Metridium senile. | Tolerant | Not relevant | Not sensitive | High |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceIf the species were to be extracted, mortality would occur. For recoverability, see additional information below. | High | High | Moderate | High |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceMetridium senile sometimes occur, as small individuals, on kelp (Laminaria hyperborea) stipes. Kelp is extracted in some countries. Where attached individuals are collected, demise is certain. For recoverability, see additional information below. | High | High | Moderate | High |
Additional information
Recovery following loss is likely to be high. The species reproduces each year and the planulae, according to Sebens (1985), spend months in the plankton and are likely to disperse over in excess of 10 km from parent anemones. New jetty piles at Lundy were colonized by their third year (L. Cole, pers. comm.). Settled planulae or individuals produced by basal laceration are likely to grow rapidly. Bucklin (1987a) found that, for Metridium senile from California, individuals showed rapid growth to large sizes when fed at frequent intervals. Mean size grew steadily during the first eight months then levelled off. An increase from 5 cm² pedal disk area to 45 cm² occurred within 12 months. However, in the clearance experiments undertaken by Sebens (1985) he found that it took 5-10 years for Metridium senile to return to pre-clearance cover rates. In another study, Wahl (1985) found that Metridium senile returned to rock walls only one week after oxic conditions returned following annual de-oxygenation events in the Inner Flensburg Fjord. Overall, a recoverability of high is probable even where no nearby populations exist.
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Metridium senile may dominate large areas of rock and artificial substrata such as jetty piles and wrecks. It is capable of aggressively displacing other species through the use of 'catch-tentacles' that sting adjacent fauna causing necrotic patches to develop. Studies of community dynamics have been undertaken particularly by K. P. Sebens (see, for instance, Sebens 1985). It is possible that several fish species may feed on Metridium senile. The sea slug Aeolidia papillosa and the sea spider Pycnogonum littorale feed on this and other sea anemones. (See 'General Biology' for more information and references.)Bibliography
Anthony, K.R.N., 1997. Prey capture by the sea anemone Metridium senile (L.): effects of body size, flow regime, and upstream neighbors. Biological Bulletin, Marine Biological Laboratory, Woods Hole, 192, 73-86.
Braber, L. & Borghouts, C.H., 1977. Distribution and ecology of Anthozoa in the estuarine region of the rivers Rhine, Meuse and Scheldt. Hydrobiologia, 52, 15-21.
Bucklin, A., 1982. The annual cycle of sexual reproduction in the sea anemone Metridium senile. Canadian Journal of Zoology, 60, 3241-3248.
Bucklin, A., 1985. Biochemical genetic variation, growth and regeneration of the sea anemone, Metridium, of British shores. Journal of the Marine Biological Association of the United Kingdom, 65, 141-157.
Bucklin, A., 1987. Growth and asexual reproduction of the sea anemone Metridium: comparative laboratory studies of three species. Journal of Experimental Marine Biology and Ecology, 110, 41-52.
Bucklin, A., 1987b. Adaptive advantages of patterns of growth and asexual reproduction of the sea anemone Metridium senile (L.) in intertidal and submerged populations. Journal of Experimental Marine Biology and Ecology, 10, 225-243.
Bull, H.O., 1939b. The Anthozoa of the Cullercoats District. Report of the Dove Marine Laboratory, 3rd Series, 6, 29.
Fautin, D.G., 2016b. Catalog to families, genera, and species of orders Actiniaria and Corallimorpharia (Cnidaria: Anthozoa). Zootaxa, 4145 (1), 1-449. DOI https://doi.org/10.11646/zootaxa.4145.1.1
Gorzula, S. & Cameron, K. 1976. A population explosion of Aeolidia papillosa at Keppel Pier, Millport, Isle of Cumbrae. Western Naturalist, 5, 67-69.
Graham, A., 1988. Molluscs: prosobranchs and pyramellid gastropods (2nd ed.). Leiden: E.J. Brill/Dr W. Backhuys. [Synopses of the British Fauna No. 2]
Griffiths, C.L., Kruger, L.M. & Smith, C.E. 1996. First record of the sea anemone Metridium senile from South Africa. South African Journal of Zoology, 31, 157-158.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hiscock, K., 1983. Water movement. In Sublittoral ecology. The ecology of shallow sublittoral benthos (ed. R. Earll & D.G. Erwin), pp. 58-96. Oxford: Clarendon Press.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Keats, D.W., 1990. Food of the winter flounder Pseudopleuronectes americanus in a sea urchin dominated community in eastern Newfoundland. Marine Ecology Progress Series, 60, 13-22.
Manuel, R.L., 1988. British Anthozoa. Synopses of the British Fauna (New Series) (ed. D.M. Kermack & R.S.K. Barnes). The Linnean Society of London [Synopses of the British Fauna No. 18.]. DOI https://doi.org/10.1002/iroh.19810660505
Mattacola, A. D., 1976. An unusual diet for bream. Journal of the Marine Biological Association of the United Kingdom, 56, 810.
MBA (Marine Biological Association), 1957. Plymouth Marine Fauna. Plymouth: Marine Biological Association of the United Kingdom.
Perron, F. 1978. The habitat and feeding behaviour of the wentletrap Epitonium greenlandicum.
Reidy, S. 1996. Comparison of associations of the nudibranch Aeolidia papillosa with two sea anemones Urticina crassicornis and Metridium senile. In Proceedings of the 24th Annual Benthic Ecology Meeting, Columbia, South Carolina, March 7-10, 1996 (ed. S.A. Woodin et al.), pp. 68.
Sebens, K.P., 1985. Community ecology of vertical rock walls in the Gulf of Maine: small-scale processes and alternative community states. In The Ecology of Rocky Coasts: essays presented to J.R. Lewis, D.Sc. (ed. P.G. Moore & R. Seed), pp. 346-371. London: Hodder & Stoughton Ltd.
Shumway, S.E., 1978. Activity and respiration of the sea anemone, Metridium senile (L.) exposed to salinity fluctuations. Journal of Experimental Marine Biology and Ecology, 33, 85-92.
Svane, I. & Groendahl, F., 1988. Epibioses of Gullmarsfjorden: an underwater stereophotographical transect analysis in comparison with the investigations of Gislen in 1926-29. Ophelia, 28, 95-110.
Wahl, M., 1984. The fluffy sea anemone Metridium senile in periodically oxygen depleted surroundings. Marine Biology, 81, 81-86.
Wahl, M., 1985. The recolonization potential of Metridium senile in an area previously depopulated by oxygen deficiency. Oecologia, 67, 255-259.
Wilhelm, E., Bueckman, D. & Tomaschko, K.-H., 1997. Life cycle and population dynamics of Pycnogonum litorale. Marine Biology, 129, 601-606.
Williams, R.B., 1975. Catch-tentacles in sea anemones: occurrence in Haliplanella luciae (Verrill) and a review of current knowledge. Journal of Natural History, 9, 241-248.
Datasets
Centre for Environmental Data and Recording, 2018. IBIS Project Data. Occurrence dataset: https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Isle of Wight Local Records Centre, 2017. IOW Natural History & Archaeological Society Marine Invertebrate Records 1853- 2011. Occurrence dataset: https://doi.org/10.15468/d9amhg accessed via GBIF.org on 2018-09-27.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Lancashire Environment Record Network, 2018. LERN Records. Occurrence dataset: https://doi.org/10.15468/esxc9a accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2018. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset: https://doi.org/10.15468/aru16v accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2022. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset:https://doi.org/10.15468/aru16v accessed via GBIF.org on 2024-09-27.
Merseyside BioBank., 2018. Merseyside BioBank (unverified). Occurrence dataset: https://doi.org/10.15468/iou2ld accessed via GBIF.org on 2018-10-01.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
Norfolk Biodiversity Information Service, 2017. NBIS Records to December 2016. Occurrence dataset: https://doi.org/10.15468/jca5lo accessed via GBIF.org on 2018-10-01.
North East Scotland Biological Records Centre, 2017. NE Scotland other invertebrate records 1800-2010. Occurrence dataset: https://doi.org/10.15468/ifjfxz accessed via GBIF.org on 2018-10-01.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-07-26
South East Wales Biodiversity Records Centre, 2023. SEWBReC Marine and other Aquatic Invertebrates (South East Wales). Occurrence dataset:https://doi.org/10.15468/zxy1n6 accessed via GBIF.org on 2024-09-27.
Suffolk Biodiversity Information Service., 2017. Suffolk Biodiversity Information Service (SBIS) Dataset. Occurrence dataset: https://doi.org/10.15468/ab4vwo accessed via GBIF.org on 2018-10-02.
The Wildlife Information Centre, 2018. TWIC Biodiversity Field Trip Data (1995-present). Occurrence dataset: https://doi.org/10.15468/ljc0ke accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 11/06/2007