Dabberlocks (Alaria esculenta)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Dr Harvey Tyler-Walters | Refereed by | Dr Stefan Kraan |
| Authority | (Linnaeus) Greville, 1830 | ||
| Other common names | - | Synonyms | Alaria platyrhiza (Linnaeus) Greville, 1830 |
Summary
Description
Short cylindrical stipe (exceptionally up to 75 cm) continuing as a distinct midrib throughout the length of the narrow, ribbon-like, slightly wavy blade. Attached to substrate by claw-like holdfast termed haptera. The blade is yellowish, olive-green or rich brown in colour, supple to the touch and very flexible. Blade length varies seasonally but is usually between 30 cm - 1.5 m (exceptionally 4 m) in length. Blade may be tattered and torn by wave action sometimes leaving only the midrib at which point it may be confused with Chorda filum. Older plants may have flat, finger-like sporophylls, each up to 10 cm in length, growing from the stipe at the base of the blade. The sporophylls bear reproductive bodies called sori. When fertile the sori form a typical H-shaped figure on the sporophylls.
Recorded distribution in Britain and Ireland
Found around the Shetland Isles, Orkney and east coast of Scotland, south to Flamborough Head in England. Its distribution continues along the south west of England and the west coasts of England, Wales, Scotland and Ireland including the Isle of Man.Global distribution
Alaria esculenta occurs in the North Atlantic from Novaya Zemlya to Iceland and south to Brittany in the east and from the shores of Greenland to the Bering Strait in the west. It also occurs in the Bering Sea and Sea of Japan in the North PacificHabitat
Alaria esculenta is found at low water and in the subtidal to about 8 m depth on exposed rocky shores. In exceptionally high exposure (e.g. Rockall, UK and Scellig Islands, Ireland) it has been recorded to 35 m depth.Depth range
0-8 mIdentifying features
- Plant with claw shaped holdfast, cylindrical stipe and flattened ribbon-like blade with distinct midrib.
- No side veins in blade.
- Un-branched (except for sporophylls near base).
- Sporophylls have a narrow base and are widest at the tip; the rounded tips often slightly expanded.
Additional information
Other common names include wing kelp, honeyware, edible fucus, and bladder locks in England; dabberlocks and keys in Scotland; and murlins, ribini, and Cupog nag Cloc in Ireland (Guiry 2000). The species name Alaria esculenta literally means 'edible wings'. This species was originally described as Fucus esculentus Linnaeus, 1767. The class Phaeophyceae may alternatively be classified in the Phylum Heterokontophyta ( Hoek van den et al. 1995).
Alaria (Phaeophyceae, Alariaceae) is a common genus of kelps in the northern hemisphere. Fourteen species are currently recognised of which three (Alaria esculenta (L.) Greville, Alaria pylaii (Bory de Saint-Vincent) Greville, and Alaria grandifolia J. Agardh) are reported for the cold -temperate North Atlantic Ocean. Alaria esculenta, the type species described originally from the North Atlantic, exhibits a range of biogeographically correlated morphotypes suggesting the possibility of multiple specific or intraspecific entities or hybrids (Kraan pers. comm.; Kraan & Guiry 2000 in press). A key to the species of the genus Alaria is given by Widdowson (1971).
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Ochrophyta | Brown and yellow-green seaweeds |
| Class | Phaeophyceae | |
| Order | Laminariales | |
| Family | Alariaceae | |
| Genus | Alaria | |
| Authority | (Linnaeus) Greville, 1830 | |
| Recent Synonyms | Alaria platyrhiza (Linnaeus) Greville, 1830 | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | |||
| Male size at maturity | |||
| Female size range | Large(>50cm) | ||
| Female size at maturity | |||
| Growth form | Straplike / Ribbonlike | ||
| Growth rate | 20 cm/month | ||
| Body flexibility | High (greater than 45 degrees) | ||
| Mobility | Sessile, permanent attachment | ||
| Characteristic feeding method | Autotroph | ||
| Diet/food source | Photoautotroph | ||
| Typically feeds on | Not relevant | ||
| Sociability | Not relevant | ||
| Environmental position | Epifloral, Epilithic | ||
| Dependency | Not relevant. | ||
| Supports | Substratum Bryozoa and several epiphytes including Litosiphon laminariae (Kraan pers. comm.). | ||
| Is the species harmful? | No Edible | ||
Biology information
- Alaria esculenta forms the main canopy in exposed rocky areas.
- Alaria esculenta is a colonizing species that will occur in recently denuded rock surfaces in exposed to sheltered situations.
- Maximum growth rates in the field (20 cm/month) occur in April and May (Isle of Man). Plants on adjacent rope aquaculture system had an average growth rate of 5 cm per day (Birkett et al., 1999b). Kain & Dawes (1987) reported growth rates of 10 cm per day during one spring growth period on a rope aquaculture system in the Isle of Man. However, fields trials with different strains of Alaria esculenta resulted in highest growth rates of 25 cm/month and lowest rates of 5 cm/month (Kraan pers. comm.).
- In June and July growth slows and the blade becomes eroded and damaged by wave action depending on depth. Alaria esculenta in the lower eulittoral and upper sublittoral will erode away completely due to higher summer water temperatures and bleaching by sunlight. Populations at 2 m or more below low water survive (Kraan pers. comm.).
- In strong currents and low wave action the blade may reach 4 m in length (e.g. Aran Islands, Ireland; Guiry, 1997).
- A short stipe and narrow lamina base is characteristic of exposed conditions whereas in sheltered conditions the stipe is long and the lamina base wider (Widdowson, 1971).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast, Sea loch or Sea lough |
| Biological zone preferences | Lower eulittoral, Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Artificial (man-made), Bedrock, Cobbles, Large to very large boulders, Pebbles, Small boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Exposed, Extremely exposed, Very exposed |
| Salinity preferences | Full (30-40 psu) |
| Depth range | 0-8 m |
| Other preferences | -2 °C winter isotherm (as far as sea ice) and up to 16 °C summer isotherm. |
| Migration Pattern | Non-migratory or resident |
Habitat Information
Alaria esculenta is absent from most of the east coast of England, primarily due to a lack of suitable substrata. The species is found all around the Irish coast, where rocky shores as substratum are available. It occurs at a depth of 15 m in the Aran Islands and below 35 m off the Scellig Islands, Ireland and Rockall.Alaria esculenta is present in the North Pacific and North Atlantic, where it is located north as far as the winter sea ice and as far south as the 16 °C summer isotherm, represented by the French coast of Brittany in the European North Atlantic (Luning, 1990). Its absence in the southern North Sea and English Channel is due to high summer surface temperatures of 16 °C, which it cannot survive (Munda & Luning, 1977; Widdowson, 1971; Sundene, 1962). Its distribution in the Arctic Sea is associated with the -2 °C February winter isotherm (Kraan pers. comm.).
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Alternation of generations |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | >1,000,000 |
| Generation time | 1 year |
| Age at maturity | 8 - 14 months |
| Season | October - May |
| Life span | 5-10 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Spores (sexual / asexual) |
| Duration of larval stage | 1 day |
| Larval dispersal potential | 10 -100 m |
| Larval settlement period |
Life history information
The fecundity reported above is for zoospore production. Both the sporophyte and gametophyte are photoautotrophs but only the gametophyte may develop vegetatively.- Dispersal potential varies with phase in the life cycle; zoospores may disperse <2m whereas gametophytes may disperse between 1 -10 m by drifting, maybe up to 100 m (Kraan pers. comm.).
- Alaria esculenta is a perennial and lives for 4 -5 years in the Irish Sea and up to 7 years in Norway.
- Two rows of ligulate sporophylls form in the upper parts of the stipe during spring and in a lesser amount in autumn only (Kraan pers. comm.; Widdowson, 1971).
- New sporophytes appear in early spring and zoospores are produced from sporophylls between October and May (Kraan pers. comm.; Birkett et al., 1998b).
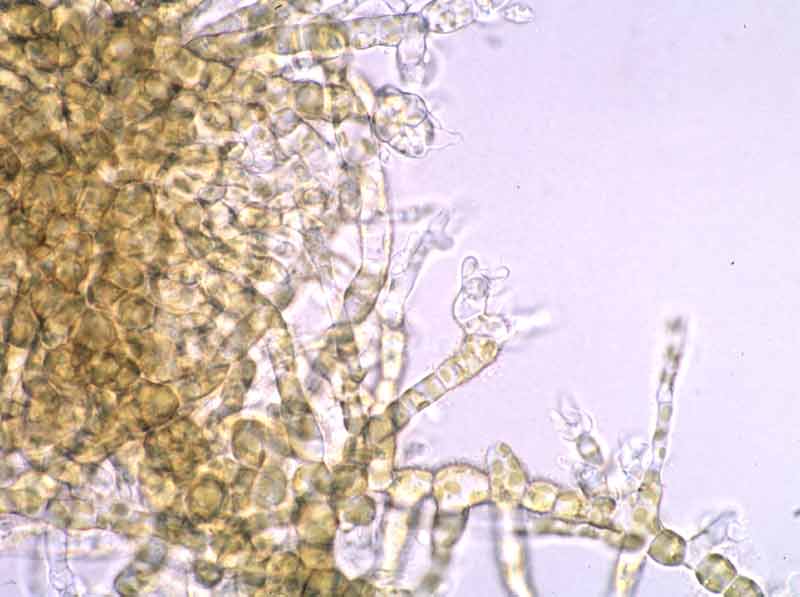
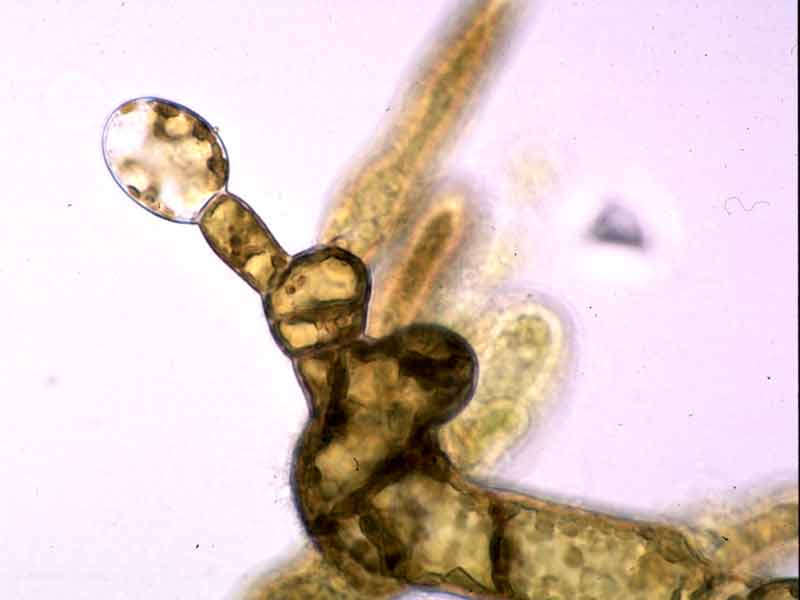
- The sporophylls produce haploid spores (zoospores), by meiosis, that germinate to form the haploid or gametophytic phase (male and female). Gametophytes produce the gametes (sperm and eggs) which fuse after fertilization to form a zygote. The zygotes germinates to form diploid platelets (germlings), the sporophytic phase. Thus the life history is that the large seaweed (sporophyte) alternates with a microscopic filamentous phase (Hoek van den, 1995). Therefore, Alaria esculenta is dioecious and has a heteromorphic diplohaplontic life history.
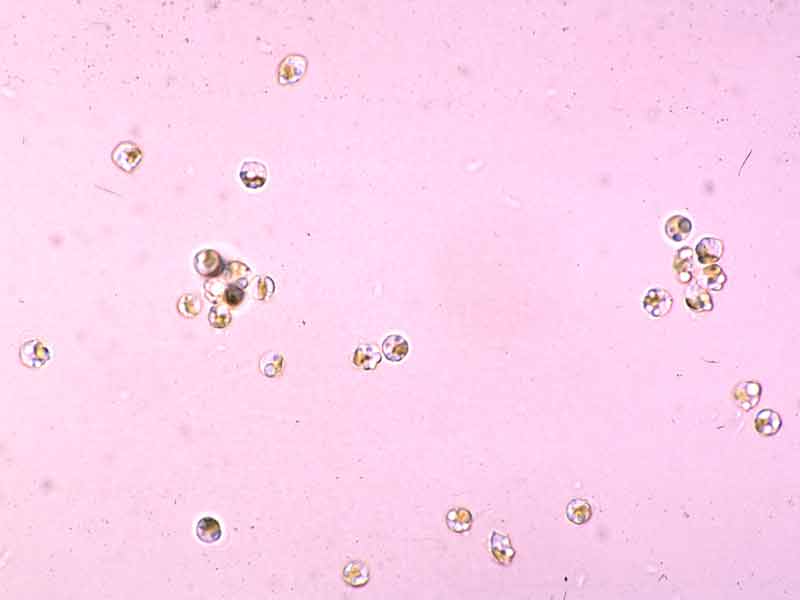
- The flagellated zoospores are about 5 microns in diameter. They loose their flagella after 24 hrs and settle on the available substrata (Birkett et al., 1998b). However, settling rate is dependant on the local currents, therefore spore settling time is probably longer than 1 day.
- Sundene (1961) noted that next generation sporophytes developed within 10 m of the parent plants in Drobak, Norway. Norton (1992) suggested that the position of the sporophylls close to the substratum may limit dispersal potential, however the local currents are probably of overriding importance for dispersal.
- Gametophytes become fertile in under 10 days in optimal conditions.
- Successful fertilization requires a high density of spore settlement (about 1 mm apart).
- Maturation of the gametophytes can be delayed under less optimal conditions, for example, low light, and development remains vegetative. Fragments of damaged vegetative gametophytes may develop into separate gametophytes (only a few cells are required) hence reproductive potential may be increased. If optimal conditions return the gametophyte may become fertile and produce gametes.
- Spore production may be inhibited by epifauna such as Membranipora membranacea (sea mat) and endophytes such as Streblonema sp.
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceRemoval of the substratum would entail removal of the plants themselves, germlings and gametophytes. They can not re-attach once removed and would be swept away. Evidence from storm damage and experimental removal of adult Laminaria species in Australia indicates that kelp forest can re-grow within 14 months. These experiments did not remove the gametophyte 'seed' bank. New individuals of Alaria esculenta colonized within 10 m of the parent plants in Norway. However, given the potentially large number of spores and gametophytes it is likely that recolonization would occur rapidly and sporophytes may grow up to 20 cm/month under optimal conditions in the field and up to 10 cm/day in rope culture systems (Birkett et al., 1998b; Kain & Dawes, 1987). | High | High | Moderate | Moderate |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceAlthough smothering of the adult sporophyte may reduce photosynthetic activity it is unlikely to cause damage. However, juvenile sporophytes may be smothered and their growth inhibited. The germlings, zoospores and gametophytes are likely to be intolerant of smothering. | Intermediate | High | Low | Low |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceIncreased sedimentation may result in smothering of adults (sporophytes), germlings and gametophytes (see above). Increased sediment deposition may increase sediment scour which may damage sporelings in particular. However, the most likely effect of increased siltation will be increased light attenuation and turbidity (see below). | Intermediate | High | Low | Low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidenceKelps are normally subtidal species and are likely to be intolerant of desiccation. Alaria esculenta may extend into the lower eulittoral in very exposed conditions. However, these marginal populations have a reduced age range in comparison to the subtidal populations due to the loss of plants resulting from sunshine at low tide. An increase in desiccation by 25% over a period of a year is likely to remove the population. | High | High | Moderate | Low |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceThe lower limit of Alaria esculenta populations is probably controlled by competition from other kelp species such as Laminaria hyperborea, Laminaria digitata and foliose red algae. A decrease in emergence is likely to extend the population up shore. However, an increase is emergence is likely to result in loss of plants at the upper limit of its distribution. Lost individuals are replaced by recruitment. However, the loss of plants will open the canopy and allow slow growing germling to replace adult sporophytes or the maturation of gametophytes. | Intermediate | High | Low | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceThe presence of Alaria esculenta is associated with strong wave action and tidal flow. Therefore, it is likely to be intolerant of any reduction in wave exposure and flow rate, which is likely to subject it to competition from other Laminarians e.g. Laminaria digitata. | Intermediate | High | Low | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceAlaria esculenta germlings tolerate up to 16 °C, above which growth is inhibited (Sundene, 1962). Sundene (1962) reported that Alaria esculenta was only found on shores where the August mean seawater surface temperature is 16 °C or lower (except in areas of extreme exposure). Munda & Lüning (1977) reported that temperatures of 16 -17 °C for a few weeks were lethal to Alaria esculenta sporophytes based on field experiments with specimens transplanted to Helgoland from north Iceland. They suggested that sporophytes were likely to survive above 16 °C for some days, but that longer duration was lethal. Temperature was, therefore, considered to be the main factor controlling its distribution in Europe (Sundene, 1962; Munda & Lüning, 1977; Widdowson, 1971; Lüning, 1990). Birkett et al. (1998b) further suggest that kelp are stenothermal (intolerant of temperature change) and that upper and lower lethal limits of kelp would be between 1-2 °C above or below the normal temperature tolerances. Given its distribution in the North Atlantic and Arctic Sea is associated with the -2 °C February winter isotherm (Kraan pers. comm.). This species is likely to be intolerant of change in temperature equivalent to either benchmark. Its southern distribution is likely to be affected by the position of the mean surface temperature isotherm in Europe and therefore climatic change. The temperature tolerances of the gametophyte stages are different to those of the adult. | High | High | Moderate | Moderate |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceThe light penetration influences the maximum depth at which kelps species can grow. Dring (1982) reported that laminarians grow at depths at which the light levels are reduced to 1% of incident light at the surface. This varies with the turbidity of the sea water from 100 m in the Mediterranean to only 6-7 m in the silt laden German Bight. Increased turbidity due to coastal engineering, dredging, cooling water plumes been reported to result in the loss of local kelp forest. Suspended material in vicinity of sewage outfalls have been reported to result in reduced the depth range and the fewer new plants under the canopy. However, Alaria esculenta is excluded from deep waters by competition from other kelp species and is unlikely to be light limited (except Rockall populations). Although likely to be intolerant of extreme increase of light attenuation it is unlikely to be affected by a long term change of turbidity of one level on the water clarity scale. | Low | High | Low | Low |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceAlaria esculenta is characteristic of wave exposed coasts and favoured by increasing exposure. However, a decrease in wave exposure is likely to subject it to competition with Laminaria spp., which are likely to replace it as the dominant species as exposure decreases | High | High | Moderate | High |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidencePlants have no known sound or vibration receptors | Tolerant | Not relevant | Not sensitive | High |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceMacroalgae are not known to react to the rapid changes in light and shade that would be associated with movement and have no known visual receptors. | Tolerant | Not relevant | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceAlaria esculenta is adapted to exposed and very wave exposed coasts. The force of wave action in these habitats is significant source of physical abrasion, which is mitigated by the flexibility of the frond. However, abrasion, for instance from a vessel stranding or equivalent disturbance, is likely to snag, damage, and remove fronds. Therefore, an intolerance of intermediate has been recorded. Recoverability is likely to be high. | Intermediate | High | Low | Moderate |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceAlaria esculenta is permanently attached to the substratum. If removed if can not re-attach (except in experimental conditions) and will be lost from the population. However, the population will recover fairly rapidly from the losses due the presence of germlings, large number of spores and gametophytes. | High | High | Moderate | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceAtrazine was lethal to young sporophytes of Laminaria hyperborea at 1 mg/l and caused growth suppression at 10 µg/l (Hopkin & Kain, 1978). Mixed detergents, herbicides (dalapon 2,4-D) were not toxic at the levels tested. Cole et al. (1999) report the following as very toxic to macrophytes: atrazine; simazine; diuron; and linuron (herbicides). It is likely therefore that Laminariales such as Alaria esculenta are intolerant of atrazine and some other herbicides. PCBs inhibited growth, gametogenesis and sporophyte recruitment in Macrocystis pyrifera at 5 µg/litre and, therefore, may have similar sub-lethal effects on Alaria esculenta | Intermediate | High | Low | Moderate |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceMercury and copper were found to be lethal at 50 µg/l and 100 µg/l respectively and toxic at 50 and 10 µg/ l respectively in Laminaria hyperborea. Zinc and cadmium were lethal at 5 mg/l and 10 mg/l respectively. Therefore, it is likely that Laminariales such as Alaria esculenta are intolerant of copper at benchmark level and so intolerance is assessed as intermediate. Zinc, cadmium and mercury especially are likely to cause sub-lethal effects. | Intermediate | High | Low | Moderate |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceMucilaginous slime coating on kelp fronds is thought to protect them from coatings of oil. Hydrocarbons in solution reduce photosynthesis and may be algicidal. Reduction in photosynthesis depends on the type of oil, its concentration, length of exposure, method used to prepare oil-water mixture and irradiance in experimental trials (Lobban & Harrison, 1994). The sublittoral fringe and lower eulittoral populations of Alaria esculenta would be most vulnerable to oiling. Subtidal populations being only exposed to oil emulsions or oil adsorbed to particles. Kelps are relatively insensitive to dispersants (Birkett et al. 1998). Three days exposure to 1% diesel emulsion reduced photosynthesis completely in young Macrocytsis plants. Laminaria digitata exposed to diesel oil at 130 µg/litre reduced growth by 50% in a two year experiment. No growth inhibition was noted at 30 µg/l and the plants recovered completed in oil-free conditions. Overall, laminarians such as Alaria esculenta are probably relatively tolerant of oiling and an intolerance of low has been recorded. | Low | Immediate | Not sensitive | Moderate |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceNo information found. | No information | No information | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceAll kelp species are efficient absorbers of nutrients (nitrates and phosphates) and can take up and store excess nutrients. In exposed sites the turnover of fresh seawater suggests that nutrients are continuously replenished. Eutrophication is associated with loss of perennial macrophytes, a reduction in the depth range and replacement by mussels or opportunistic algae species (Fletcher, 1996; Birkett et al., 1998). Increased nutrients may increase growth of epiphytes and plankton, resulting in reduced light penetration for photosynthesis and a subsequent reduction in the depth at which kelp could grow. However, nutrients are often added to macrophyte cultures to increase productivity. Therefore a rank of intermediate intolerance has been given to represent the likely indirect effects on turbidity and competition. | Intermediate | High | Low | Low |
Increase in salinity [Show more]Increase in salinity
EvidenceKelps are found in full salinity and thought to be stenohaline, i.e. intolerant of salinity change. Alaria esculenta sporophytes grew poorly at salinities below 25 psu (Sundene 1961). Although its may penetrate into the lower eulittoral in is probably highly intolerant of long term reductions in salinity (Birkett et al., 1998). | High | High | Moderate | Low |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceNo information was found concerning on the effects of de-oxygenation in macrophytes. | No information | Not relevant | No information | Not relevant |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceGalls on the blade of Laminaria hyperborea and spot disease are associated with the endophyte Streblonema sp. although the causal agent is unknown (bacteria, virus or endophyte). Resultant damage to the blade and stipe may increase losses in storms. The endophyte inhibits spore production and therefore recruitment and recoverability. Streblonema sp. has been reported growing on Alaria esculenta but no further information was found (Lein et al., 1991). | Low | Immediate | Not sensitive | Moderate |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceThe Japanese kelp Undaria pinnatifida (wakame) has recently spread to the south coast of England from Brittany where it was introduced for aquaculture. It is presently restricted to man made structures but could spread in the ballast water of commercial or recreational boats and shipping. Its potential competition with other kelps in the UK, including Alaria esculenta requires further study (Birkett et al., 1998). | Low | Immediate | Not sensitive | Low |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceThere is considerable material on the effects of harvesting kelp species (Birkett et al. 1998; Guiry & Blunden, 1991) but little evidence concerning the effects of harvesting on Alaria esculenta populations. However, evidence from other kelp species suggest that the macrophytes can recover within 3-4 years, although the effects on the rest of the community is poorly studied. In canopy clearance experiments, Alaria esculenta may appear early in the succession suggesting that it would recover more rapidly. | Intermediate | High | Low | Low |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceExtraction of sea urchin predators such as lobsters and sea otters in Nova Scotia was suggested as a possible cause of catastrophic sea urchin infestation and loss of kelp forest. Removal of grazing species may reduce competition with urchins leading to a increase in their population and subsequent loss of kelp species. However, present evidence indicates that periodic good recruitment to the urchin population may have a greater effect. It seem likely that each of these factors influences urchin populations to different extents in different areas. These effects are poorly studied in UK populations of kelp. An increased urchin or other grazer population may increase time taken for the population to recover, since they are likely to remove young sporophytes. | Intermediate | Moderate | Moderate | Low |
Additional information
The ecophysiology and chemical tolerances of Alaria esculenta is poorly studied. Most of the information presented here is based on work on other Laminariales. The effects of pollutants on macrophytes, including Laminariales, is reviewed by Loban and Harrison (1997).
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
- Alaria esculenta was used in the past in both Scotland and Ireland for human consumption and fodder. It was also gathered and spread on infertile land as fertilizer (Newton, 1931; Guiry & Hession, 1996; Guiry, 1997; Guiry & Blunden, 1991).
- It is rich in sugars, proteins, vitamins and other trace metals and contains up to 42% alginic acid (Levring et al., 1969; Indergaard & Minsaas, 1991; Lewallen & Lewallen, 1996).
- The species can be used for a variety of purposes from human consumption and alginate production to fodder and bodycare products (Guiry & Blunden, 1991; Guiry, 1997). In North America especially, Alaria esculenta and Alaria marginata are rapidly gaining popularity in the natural foods market (Lewallen & Lewallen, 1996).
- Alaria esculenta also has potential as a foodstuff in aquaculture for herbivorous molluscs, e.g. the abalone (Mai et al., 1996).
- Young Alaria esculenta (the only native species in Ireland) can be used as a substitute for Undaria pinnatifida (Wakame), a very popular seaweed in Asian countries with numerous applications and a yield of over 300,000 tonnes fresh weight per annum (Nisizawa et al., 1987; Indergaard & Minsaas, 1991; Druehl, 1988; Yamanaka & Akiyama, 1993; Lewallen & Lewallen, 1996).
- Field experiments in the Isle of Man have shown that a harvest of 9 tonnes per hectare is possible within 3 months. Alaria esculenta is a fast growing seaweed and can grow at up to 10 cm/day. Commercial potential for Alaria esculenta is considerable (Kain & Dawes 1987; Kain et al. 1990).
Bibliography
Birkett, D.A., Maggs, C.A., Dring, M.J. & Boaden, P.J.S., 1998b. Infralittoral reef biotopes with kelp species: an overview of dynamic and sensitivity characteristics for conservation management of marine SACs. Natura 2000 report prepared by Scottish Association of Marine Science (SAMS) for the UK Marine SACs Project., Scottish Association for Marine Science. (UK Marine SACs Project, vol VI.), 174 pp. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/reefkelp.pdf
Connor, D.W., Dalkin, M.J., Hill, T.O., Holt, R.H.F. & Sanderson, W.G., 1997a. Marine biotope classification for Britain and Ireland. Vol. 2. Sublittoral biotopes. Joint Nature Conservation Committee, Peterborough, JNCC Report no. 230, Version 97.06., Joint Nature Conservation Committee, Peterborough, JNCC Report no. 230, Version 97.06.
Dieck, T.I., 1993. Temperature tolerance and survival in darkness of kelp gametophytes (Laminariales: Phaeophyta) - ecological and biogeographical implications. Marine Ecology Progress Series, 100, 253-264.
Dring, M.J., 1982. The Biology of Marine Plants. London: Edward Arnold.
Druehl, L., 1988. Laminaria aquaculture in British Columbia. In Proceedings of the Fourth Alaska Aquaculture Conference, November 18-21, 1987, Sitka, Alaska USA, (Alaska Sea Grant Report, No. 88-4.), pp. 3-10.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Fletcher, R.L., 1996. The occurrence of 'green tides' - a review. In Marine Benthic Vegetation. Recent changes and the Effects of Eutrophication (ed. W. Schramm & P.H. Nienhuis). Berlin Heidelberg: Springer-Verlag. [Ecological Studies, vol. 123].
Guiry, M.D. & Blunden, G., 1991. Seaweed Resources in Europe: Uses and Potential. Chicester: John Wiley & Sons.
Guiry, M.D. & Hession, C., 1996. Eat up your seaweed. Ireland of the Welcomes, 45, 22-25.
Guiry, M.D. & Nic Dhonncha, E., 2000. AlgaeBase. World Wide Web electronic publication http://www.algaebase.org, 2000-01-01
Guiry, M.D., 1997. Research and development of a sustainable Irish seaweed industry. Occasional Papers in Irish Science and Technology, No. 14, Went Memorial Lecture 1996., Royal Dublin Society, Dublin, 11pp.
Hardy, F.G. & Guiry, M.D., 2003. A check-list and atlas of the seaweeds of Britain and Ireland. London: British Phycological Society
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Hiscock, S., 1979. A field key to the British brown seaweeds (Phaeophyta). Field Studies, 5, 1- 44.
Hopkin, R. & Kain, J.M., 1978. The effects of some pollutants on the survival, growth and respiration of Laminaria hyperborea. Estuarine and Coastal Marine Science, 7, 531-553.
Indergaard, M. & Minsaas, J., 1991. Animal and Human Nutrition. In Seaweed Resources in Europe: Uses and Potential, (Ed. M.D. Guiry & G. Blunden). Chichester: John Wiley & Sons.
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Kain, J.M. & Dawes, C.P., 1987. Useful European seaweeds: past hopes and present cultivation. Hydrobiologia, 151/152, 173-181.
Kain, J.M., Holt, T.J. & Dawes, C.P., 1990. European Laminariales and their cultivation. In Economically important marine plants of the Atlantic: their biology and cultivation, (ed. C. Yarish, C.A. Penniman and P. van Patten). Connecticut Sea Grant College Program. Groton.
Kraan S. & Guiry M.D., 2001. Are North Atlantic Alaria esculenta and A. grandifolia (Alariaceae, Phaeophyceae) conspecific? European Journal of Phycology, 36(1), 35-42
Lein, T.E., Sjøtun, K. & Wakili, S., 1991. Mass-occurrence of a brown filamentous endophyte in the lamina of the kelp Laminaria hyperborea (Gunnerus) Foslie along the southwestern coast of Norway. Sarsia, 76 (3), 187-193. DOI https://doi.org/10.1080/00364827.1991.10413474
Levring, T., Hoppe, H.A. & Schmid, O.J., 1969. Marine Algae: a survey of research and utilization. Hamburg: Cram, de Gruyter & Co. [Botanica Marina Handbooks, Vol. 1.]
Lewallen, E. & Lewallen, J., 1996. Sea vegetable gourmet cookbook and wildcrafter's guide. California: Mendocino Sea Vegetable Company.
Lobban, C.S. & Harrison, P.J., 1997. Seaweed ecology and physiology. Cambridge: Cambridge University Press.
Madlener, J.C., 1977. The sea vegetable book. New York: Clarkson N. Potter.
Mai, K.S., Mercer, J.P. & Donlon, J., 1996. Comparative studies of on the nutrition of 2 species of Abalone, Haliotis tuberculata and Haliotis discus Hanni-Ino. 5. The role of poly-unsaturated fatty acids in macroalgae in Abalone nutrition. Aquaculture, 139, 77-89.
Munda, I.M. & Luning, K., 1977. Growth performance of Alaria esculenta off Helgoland. Helgolander Wissenschaftliche Meeresuntersuchungen, 29, 311-314.
Newton, L., 1931. A handbook of the British seaweeds. London: British Museum (Natural History).
Nisizawa, K., Noda, H., Kikuchi, R., Watanabe, T., 1987. The main seaweed foods in Japan. Hydrobiologia, 151/152, 5-29.
Norton, T.A., 1992. Dispersal by macroalgae. British Phycological Journal, 27, 293-301.
Picton, B.E. & Costello, M.J., 1998. BioMar biotope viewer: a guide to marine habitats, fauna and flora of Britain and Ireland. [CD-ROM] Environmental Sciences Unit, Trinity College, Dublin.
Stein, F., Sjotun, K., Lein, T.E. & Rueness, J., 1995. Spore dispersal in Laminaria hyperborea (Laminariales, Phaeophyceae) Sarsia, 80, 47-53.
Sundene, O., 1962. The implications of transplant and culture experiments on the growth and distribution of Alaria esculenta. Nytt Magasin for Botanik, 9, 155-174.
Widdowson, T.B., 1971. A taxonomic revision of the genus Alaria Greville. Syesis, 4, 11-49.
Yamanaka, R. & Akiyama, K., 1993. Cultivation and utilization of Undaria pinnatifida (wakame) as food. Journal of Applied Phycology, 5, 249-253.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2014. Occurrence dataset: https://doi.org/10.15468/erweal accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2016. Occurrence dataset: https://doi.org/10.15468/146yiz accessed via GBIF.org on 2018-09-27.
Manx Biological Recording Partnership, 2017. Isle of Man wildlife records from 01/01/2000 to 13/02/2017. Occurrence dataset: https://doi.org/10.15468/mopwow accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2018. Isle of Man historical wildlife records 1995 to 1999. Occurrence dataset: https://doi.org/10.15468/lo2tge accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2022. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset:https://doi.org/10.15468/aru16v accessed via GBIF.org on 2024-09-27.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-08
Outer Hebrides Biological Recording, 2018. Non-vascular Plants, Outer Hebrides. Occurrence dataset: https://doi.org/10.15468/goidos accessed via GBIF.org on 2018-10-01.
Royal Botanic Garden Edinburgh, 2018. Royal Botanic Garden Edinburgh Herbarium (E). Occurrence dataset: https://doi.org/10.15468/ypoair accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 29/05/2008