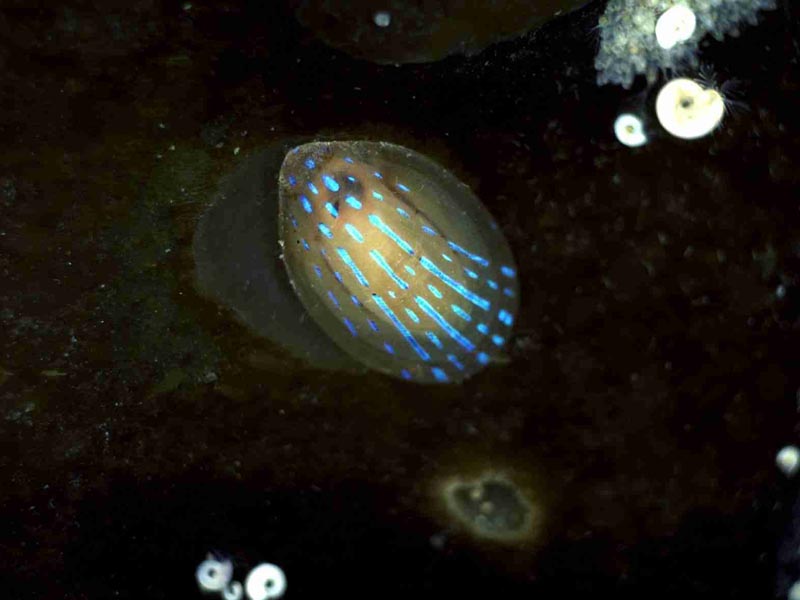
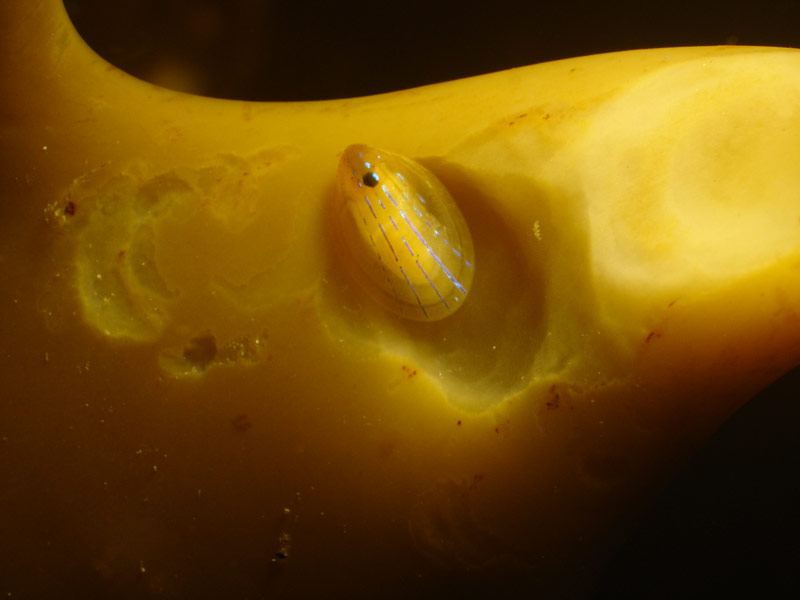
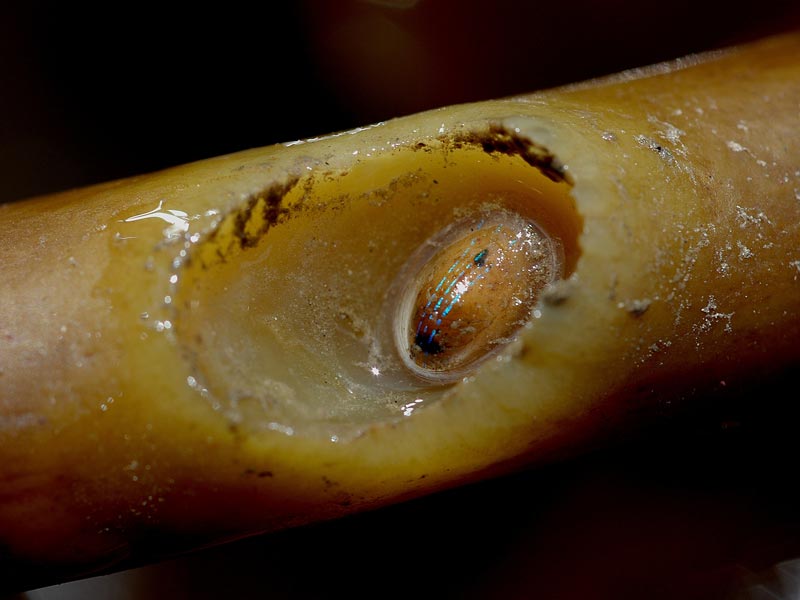
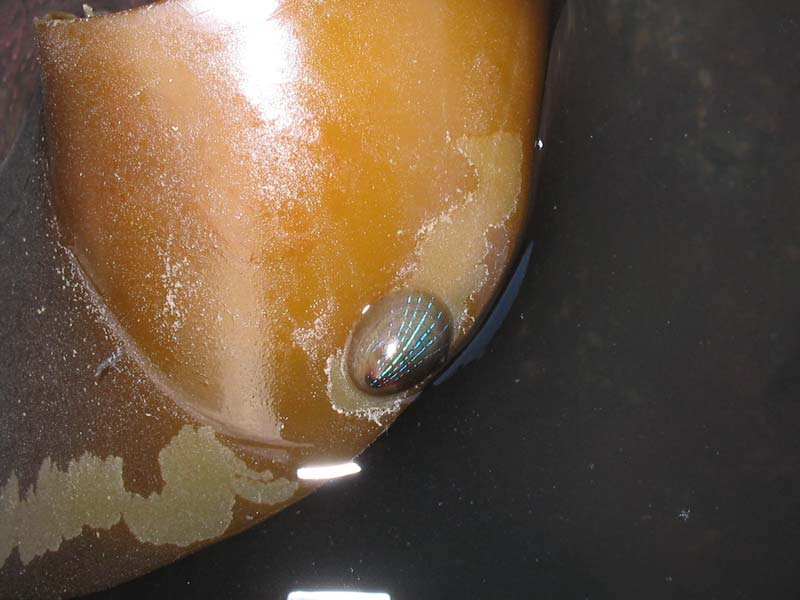
Blue-rayed limpet (Patella pellucida)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Dr Harvey Tyler-Walters | Refereed by | Dr David McGrath |
| Authority | Linnaeus, 1758 | ||
| Other common names | - | Synonyms | Patina laevis , Patina pellucida Linnaeus, 1758, Helcion laevis , Helcion pellucidum |
Summary
Description
Smooth, translucent brownish yellow - horn coloured shell, up to 2 cm in length and much smaller than the common limpet (Patella spp.). Oval in outline in the shape of a depressed cone with the apex at the anterior end. The shell is overlayed by 2-8, characteristic, broken (dashed) blue-green rays, although these are absent from the juvenile shell until they reach 1.1mm in length.
Recorded distribution in Britain and Ireland
Recorded on all coasts of Britain and Ireland, except the coast surrounding the Wash.Global distribution
Occurs in Iceland and from north Norway to south Portugal. It is found on the west coasts of Denmark, and Sweden south to Oresund. However it is absent from the Baltic sea and the east coasts of Denmark, Belgium and Holland.Habitat
Found on the blade of laminarians or fronds of Fucus serratus, Mastocarpus stellatus or Himanthalia elongata from the lower eulittoral to a depth of ca 27 m. Newly settled juveniles found on encrusting coralline algae in wave exposed conditions in the lower eulittoral. It prefers areas of considerable water flow and is not normally found in areas of low flow, siltation or freshwater influence.Depth range
ca +1 m - ca 25 mIdentifying features
- Small, translucent, brownish yellow - horn coloured depressed shell.
- Characteristic dashed blue rays radiating from apex of shell.
- Foot and body cream but browner on head and edge of foot
- No pallial gills on edge of mantle at front of body
Additional information
Weber et al. (1997) suggested that Patella pellucida (as Helcion pellucidum) was genetically distinct from its South African con-geners and may have arisen independently. Studies of morphological features (Ridgeway et al., 1998) and molecular characteristics (Koufopanou et al., 1999) suggested that Helcion pelludicum belonged to the genus Patella and the species name was restored to Patella pellucida (Linnaeus, 1758).
Specimens found in cavities in holdfasts develop into the laevis form (Patella pellucida var. laevis). The laevis form has a taller, more robust opaque shell, ledged in profile, with blue rays that alternate with reddish brown rays. The most noticeable ledge of the shell indicates the size at which the individual enters the holdfast.
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Mollusca | Snails, slugs, mussels, cockles, clams & squid |
| Class | Gastropoda | Snails, slugs & sea butterflies |
| Family | Patellidae | |
| Genus | Patella | |
| Authority | Linnaeus, 1758 | |
| Recent Synonyms | Patina laevis Patina pellucida Linnaeus, 1758Helcion laevis Helcion pellucidum | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Moderate density | ||
| Male size range | 3 - 12mm | ||
| Male size at maturity | 5mm | ||
| Female size range | 5mm | ||
| Female size at maturity | |||
| Growth form | |||
| Growth rate | 1-2mm/month | ||
| Body flexibility | Low (10-45 degrees) | ||
| Mobility | Creeper | ||
| Characteristic feeding method | Grazer | ||
| Diet/food source | Herbivore | ||
| Typically feeds on | %Laminaria hyperborea%, %Laminaria digitata%, %Alaria esculenta%, %Saccorhiza polyschides%, %Fucus serratus%, and when young %Himanthalia elongata% and %Mastocarpus stellatus%. | ||
| Sociability | No information | ||
| Environmental position | Epifaunal | ||
| Dependency | Not relevant. | ||
| Supports | Not relevant | ||
| Is the species harmful? | No No text entered | ||
Biology information
Growth is rapid in summer, autumn and winter but slow in winter. Population studies suggest that few individuals survive to their second year (Fretter & Graham 1976; Graham & Fretter 1947). Vahl (1971) noted growth irregularities or 'checks' in the shell of specimens from Norway, which he suggested were caused by the interrupted growth of the mantle edge when the adult was retracted in response to severe wave action during heavy storms.
Adults can recolonize vacant fronds (McGrath, 1997), perhaps via the surface of the substratum or by mucus rafting, and if dislodged adults can right themselves and be carried to neighbouring plants by currents by secreting a mucus 'sail' (Vahl 1983).
Kain & Svendsen (1969) provide pictures of Patella pellucida on blades of Laminaria hyperborea together with the cavities grazed in the fronds and in holdfasts. Kain & Svendsen (1969) noted that in Norwegian populations severe grazing by Patella pellucida may result in perforation of blades by autumn (before new blades develop) and in some cases grazing where the blade and stipe meet may 'cut off' the blade.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast, Strait or Sound, Sea loch or Sea lough, Open coast, Sea loch or Sea lough, Strait or Sound |
| Biological zone preferences | Lower eulittoral, Sublittoral fringe, Upper infralittoral, Lower eulittoral, Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Macroalgae, Macroalgae |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Moderately strong 1 to 3 knots (0.5-1.5 m/sec.) |
| Wave exposure preferences | Exposed, Moderately exposed, Exposed, Moderately exposed |
| Salinity preferences | Full (30-40 psu), Full (30-40 psu) |
| Depth range | ca +1 m - ca 25 m |
| Other preferences | Occurs at 15 psu in Norway. |
| Migration Pattern |
Habitat Information
Studies in south-east Ireland demonstrated that the distribution of Patella pellucida on the shore depended on its size and age. McGrath (1992) noted that spat settled with a larval shell attached of ca 0.66 mm. Newly settled spat have a preference for lower shore Lithothamnia (encrusting coralline algae) reaching densities as high as 400 per sq. dm in February. As they grow juveniles (up to 1.8 mm) migrate to Mastocarpus stellatus. The juveniles recruit to Laminarians at about 1.8 mm but are found mainly at the tips of the fronds. Juveniles up to 3 mm may also be found on the receptacles of Himanthalia elongata. Dense populations may be found on Fucus serratus, Alaria esculenta, Palmaria palmata and Halidrys siliquosa (McGrath, 1992).Adults show a seasonal migration on Laminaria hyperborea, migrating down to the stipe before the old blade tissue is discarded in spring to early summer. Larger individuals prefer the lower wave exposure of deeper water (Warburton 1976).
Approximately one third of the population examined by Graham & Fretter (1947) were the laevis form. However, Kain & Svendsen (1969) did not find any specimens in Laminarian holdfasts in Norwegian populations and the laevis form may be absent in Norway.Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | Annual protracted |
| Fecundity (number of eggs) | No information |
| Generation time | <1 year |
| Age at maturity | circa 6 months (5mm in size) |
| Season | Insufficient information |
| Life span | 1-2 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | Veliger |
| Larval/juvenile development | Planktotrophic |
| Duration of larval stage | 11-30 days |
| Larval dispersal potential | 10 -100 m |
| Larval settlement period | Insufficient information |
Life history information
Few individuals survive into their second years. Most specimens > 1 year old are found in holdfasts as the laevis form. Breeding occurs throughout the year with a peak in spring. Fertilization is external and eggs are shed singly. The eggs are greenish, ca 0.16 mm across and covered with a gelatinous coat giving an overall diameter of ca 0.32 mm (Fretter & Graham, 1976; Lebour, 1937). Eggs hatch into a 200 micrometer tall trochophore that develops into a 160-180 micrometer veliger larva (Lebour, 1937). Fretter & Graham (1947) state that planktonic life is 'a few weeks'. There is little information on dispersal range, however, 10-100m is assumed given the depth of adult distribution and its settlement on lower shore at least. McGrath (1992) examined recruitment in south east Ireland and reported that newly settled spat have a preference for lower shore Lithothamnia (encrusting corallines). As they grow juveniles (up to 1.8 mm) migrate to Mastocarpus stellatus. The juveniles recruit to Laminarians at about 1.8 mm but are found mainly at the tips of the fronds. Juveniles up to 3mm may also be found on the receptacles of Himanthalia elongata. McGrath (1992) suggested that larvae settle on Lithothamina and migrate to Mastocarpus stellatus as they grow and finally to Laminaria sp. via Himanthalia elongata.Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceLoss of the substratum, this species food plants, will involve removal of the adults themselves. Adults may be lost with plants during storms or swept off the fronds. However, McGrath (1997) demonstrated that Patella pellucida can rapidly recolonize available plants with as little as three days from adjacent plants. It is likely that recolonization from adjacent populations would be fairly rapid. | High | Very high | Low | Moderate |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceSmothering by 5 cm of material is unlikely to affect adults on the fronds of kelps. The laevis form in holdfasts may be more intolerant. Similarly, the typical food species have a low intolerance to smothering. Smothering is likely to interfere with the settlement of larvae which, if lost, may significantly reduce the population of this near annual species. | Intermediate | Very high | Low | Low |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidencePatella pellucida is not found in areas of low water flow and siltation. Increased levels of suspended sediment are likely to interfere with feeding. However, its typical food species has a low intolerance to siltation. Larvae may be more intolerant at settlement which, if lost, may significantly reduce the population of this near annual species. | Intermediate | Very high | Low | Low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidenceSubtidal adults are unlikely to be affected except at extreme low tides. Increased desiccation is likely to affect the juvenile stages found in the lower eulittoral and adults on Fucus serratus. These are likely to be intolerant of an increased desiccation equivalent to moving from the lower to mid eulittoral for a year. Although the limpet can close tightly to macroalgae and creates pits and scars like its littoral co-familial Patella spp. the typical food plants (its substratum) are likely to be intolerant of increased desiccation and increased competition from other algal species more tolerant of desiccation. However, it is likely that recolonization from neighbouring populations would be rapid once the original conditions returned. | Intermediate | Very high | Low | Low |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceDecreased emergence is likely to increase the distribution of kelps up the shore and therefore Patella pellucida. Increasing emergence may reduce the upper extent of the kelp species, however, Patella pellucida could move to alternative food species such as Fucus serratus. | Low | Immediate | Not sensitive | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceThe distribution of Patella pellucida is dependent on water flow rate. Studies in Lough Ine rapids (Ebling et al. 1948) found that Patella pellucida was very scarce on Saccorhiza polyschides in weak currents, plentiful in moderately strong currents (0.6-1.5 m/s) and scarce in strong currents (>1.5 m/s). Warburton (1976) demonstrated that large individuals could resist currents up to 0.9-1.3 m/s and smaller individuals resisted stronger currents before being swept off the fronds of kelp. Adults aligned themselves with the current flow above 0.5m/s and currents above 1.0- 1.4 m/s interfered with feeding and normal behaviour. Larger individuals are found in higher abundance in deeper water although Fretter & Graham (1994) suggested that larger individuals migrate to holdfasts. Therefore, it is likely that this species would be intolerant of either a decrease or increase in the water flow rate, equivalent to the benchmark, outside its habitat preferences. | Intermediate | Very high | Low | Moderate |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceThe wide distribution of this species suggests that it is tolerant of a wide range of temperatures. However, no information on the temperature tolerance of this species was found. It is likely that its food species are intolerant of increases in temperature consistent with the benchmark, so an intermediate intolerance has been recorded. | Intermediate | Very high | Low | |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceTurbidity resulting from suspended sediment may interfere with feeding as above. Reduced light penetration will reduce the extent of the food species (kelps). | Intermediate | Very high | Low | |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceThis species prefers exposed to moderately exposed shores and it is likely to be intolerant of a change in wave exposure. The available food kelp species will change with exposure and increasing exposure may result in loss of older plants , especially those whose holdfasts had been weakened by Patella pellucida feeding, and adults of this species. | Low | Immediate | Not sensitive | |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidenceThere is no known effect of noise on this species or its prey species. | Tolerant | Not relevant | Not sensitive | Moderate |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceAlthough this species probably displays phototaxis there is no evidence of disturbance due to visual stimuli. | Tolerant | Not relevant | Not sensitive | Moderate |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceThe shell in this species is relatively thin when compared with other limpets. Abrasion at the benchmark level is likely to knock some individuals off its food plant and crush or fracture the shell of others. A passing scallop dredge is likely to remove its substratum, i.e. kelps resulting in substratum loss as above. Therefore, a single scallop dredge will remove or damage a proportion of the kelp canopy and hence a proportion of Patella pellucida. Therefore, intolerance has been assessed as intermediate. | Intermediate | Very high | Low | Low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceIt is presumed that individuals of this species are periodically swept off their food plant. Although some individuals may be lost to deep water, Patella pellucida can re-orientate itself (as it will land upside down, foot upper most) and move to other plants using a mucus 'sail', secreted by the glands of the foot (Vahl 1983). McGrath (1997) demonstrated that plants cleared of Patella pellucida are rapidly recolonized by adults. | Tolerant | Not relevant | Not sensitive | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceGastropod molluscs are known to be sensitive to endocrine disruption from synthetic chemicals such as tri-butyl tin. However no information on the specific effects of tri-butyl tin on Patella pellucida was found. Hoare & Hiscock (1974) reported that Patella pellucida was excluded form Amlwch Bay, Anglesey by the presence of acidified, halogenated effluent; only eight specimens being found in Amlwch harbour where the silt levels probably reduced the toxicity of chlorine. Patella pellucida probably has an intermediate intolerance to, at least, this form of pollution. | Intermediate | Very high | Low | Low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceBryan (1984) suggested that gastropods are rather tolerant of heavy metals. Crompton (1997) states that the following concentrations of heavy metals have caused mortalities in gastropods after 4-14days (short term); Cu (0.01-0.1 mg/l), Pb (0.1-1mg/l) , Zn (1-10mg/l), Cr and Ni (10-100mg/l). However, no data for this species was found. | No information | No information | No information | Not relevant |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceInsufficientinformation | No information | No information | No information | Not relevant |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficientinformation | No information | No information | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceIncreased nutrients are likely to increase epiphyte and food plant growth, potentially increasing the availability of food for Patella pellucida. However, significant increases in nutrient levels (eutrophication), resulting in excessive growth of epiphytes and phytoplankton may have a detrimental effect on this species food plants and, therefore the population of Patella pellucida. | Intermediate | Very high | Low | |
Increase in salinity [Show more]Increase in salinity
EvidencePatella pellucida is found in full salinity but not in areas of freshwater influence. Juveniles settle on the lower eulittoral and are likely to be subject to freshwater runoff and rainfall at low tide. However, adults are primarily subtidal and likely to be intolerant of long term reduction in salinity outlined in the benchmark. | Intermediate | Very high | Low | Low |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceOxygen concentrations at the level of the benchmark thought to likely to cause effects on marine organisms. In areas of exposure and moderately strong current flow it is unlikely to experience low oxygen levels. Therefore, it is likely to be intolerant of any spillage or activity that reduced the dissolved oxygen concentration to the level of the benchmark | Intermediate | Very high | Low |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceNo microbial pathogens were reported in the literature. | No information | No information | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceNo known alien or non-native species compete with Patella pellucida. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceThis species is not subject to extraction. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceKelp species are harvested in Scotland, Isle of Man and Ireland. Extraction of kelp is equivalent to removal of substratum (see above) in Patella pellucida. | High | Very high | Low | Moderate |
Additional information
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | Not relevant |
Importance information
Patella pellucida is an important and characteristic herbivore on Laminarians (kelps). It forms distinct pits in the surface of the blade or stipe. The laevis form feeds within the holdfast creating distinct cavities. Photographic images of Patella pellucida on Laminaria hyperborea are shown in Kain & Svendsen (1969). Its feeding, especially in the holdfast weakens the plant and is associated with the loss of adult plants from the kelp forest due to wave or storm action.Bibliography
Bryan, G.W., 1984. Pollution due to heavy metals and their compounds. In Marine Ecology: A Comprehensive, Integrated Treatise on Life in the Oceans and Coastal Waters, vol. 5. Ocean Management, part 3, (ed. O. Kinne), pp.1289-1431. New York: John Wiley & Sons.
Crompton, T.R., 1997. Toxicants in the aqueous ecosystem. New York: John Wiley & Sons.
Ebling, F.J., Kitching, J.A., Purchon, R.D. & Bassingdale, R., 1948. The ecology of Lough Ine rapids with special reference to water currents. 2. The fauna of the Saccorhiza canopy. Journal of Animal Ecology, 17, 223-244.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Fretter, V. & Graham, A., 1994. British prosobranch molluscs: their functional anatomy and ecology, revised and updated edition. London: The Ray Society.
Fretter, V., & Graham, A., 1976. The Prosobranch Molluscs of Britain and Denmark. Part 1. - Pleurotomariacea, Fissurellacea and Patellacea. Journal of Molluscan Studies, Supplement 1.
Graham, A. & Fretter, V., 1947. The life history of Patina pellucida. Journal of the Marine Biological Association of the United Kingdom, 26, 590-601.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hoare, R. & Hiscock, K., 1974. An ecological survey of the rocky coast adjacent to the effluent of a bromine extraction plant. Estuarine and Coastal Marine Science, 2 (4), 329-348.
Kain, J.M. & Svendsen, P., 1969. A note on the behaviour of Patina pellucida in Britain and Norway. Sarsia, 38, 25-30.
Koufopanou, V., Reid, D.G., Ridgeway, S.A., & Thomas, R.H., 1999. A Molecular Phylogeny of the Patellid Limpets (Gastropoda: Patellidae) and Its Implications for the Origins of Their Antitropical Distribution. Molecular Phylogenetics and Evolution, 11, 138-156.
Lebour, M.V., 1937. The eggs and larvae of the British Prosobranchs with special reference to those living in the plankton. Journal of the Marine Biological Association of the United Kingdom, 22, 105-166.
MBA (Marine Biological Association), 1957. Plymouth Marine Fauna. Plymouth: Marine Biological Association of the United Kingdom.
McGrath, D., 1992. Recruitment and growth of the Blue-rayed limpet, Helcion pellucidum in south east Ireland. Journal of Molluscan Studies, 58, 425-431.
McGrath, D., 1997. Colonization of artificially cleared Laminaria digitata (Huds.) Lamour, by the blue-rayed limpet Helcion pellucidum (L.) (Mollusca: Gastropoda). Biology and Environment: Proceedings of the Royal Irish Academy, 97B, 245-248.
Ridgeway, S.A., Reid, D.G., Taylor, J.D., Branch, G.M. & Hodgson, A.N., 1998. A cladistic phylogeny of the family Patellidae (Mollusca: Gastropoda). Philosophical Transactions of the Royal Society of London, Series B, 353(1375), 1645-1671.
Seaward, D.R., 1982. Sea area atlas of the marine molluscs of Britain and Ireland. Peterborough: Nature Conservancy Council.
Seaward, D.R., 1990. Distribution of marine molluscs of north west Europe. Peterborough: Nature Conservancy Council.
Vahl, O., 1971. Growth and density of Patina pellucida (L.) (Gastropoda: Prosobranchia) on Laminaria hyperborea (Gunnerus) from Western Norway. Ophelia, 9, 31-50.
Vahl, O., 1983. Mucus drifting in the limpet Helcion (=Patina) pellucidus (Prosobranchia, Patellidae). Sarsia, 68, 209-211.
Warburton, K., 1976. Shell form, behaviour and tolerance to water movement in the limpet Patina pellucida (L.) (Gastropoda: Prosobranchia). Journal of Experimental Marine Biology and Ecology, 23, 307-325
Weber, L.I., Gray, D.R., Hodgson, A.N., & Hawkins, S.J., 1997. Genetic divergence between South African Helcion species and North Atlantic Helcion pellucidum (Mollusca: Patellogastropoda). Journal of the Marine Biological Association of the United Kingdom, 77, 1139-1150.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2018. Mollusc (marine) data for Great Britain and Ireland - restricted access. Occurrence dataset: https://doi.org/10.15468/4bsawx accessed via GBIF.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2023. Mollusc (marine) records for Great Britain and Ireland. Occurrence dataset: https://doi.org/10.15468/aurwcz accessed via GBIF.org on 2024-09-27.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2014. Occurrence dataset: https://doi.org/10.15468/erweal accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
Kent Wildlife Trust, 2018. Biological survey of the intertidal chalk reefs between Folkestone Warren and Kingsdown, Kent 2009-2011. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Manx Biological Recording Partnership, 2017. Isle of Man wildlife records from 01/01/2000 to 13/02/2017. Occurrence dataset: https://doi.org/10.15468/mopwow accessed via GBIF.org on 2018-10-01.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-07-26
Outer Hebrides Biological Recording, 2018. Invertebrates (except insects), Outer Hebrides. Occurrence dataset: https://doi.org/10.15468/hpavud accessed via GBIF.org on 2018-10-01.
South East Wales Biodiversity Records Centre, 2018. SEWBReC Molluscs (South East Wales). Occurrence dataset: https://doi.org/10.15468/jos5ga accessed via GBIF.org on 2018-10-02.
The Wildlife Information Centre, 2018. TWIC Biodiversity Field Trip Data (1995-present). Occurrence dataset: https://doi.org/10.15468/ljc0ke accessed via GBIF.org on 2018-10-02.
Yorkshire Wildlife Trust, 2018. Yorkshire Wildlife Trust Shoresearch. Occurrence dataset: https://doi.org/10.15468/1nw3ch accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 08/05/2008