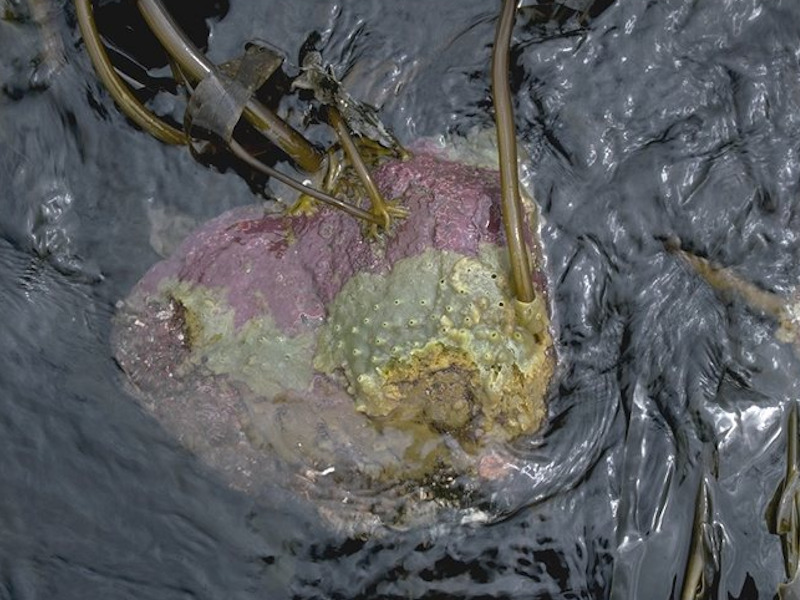
Laminaria digitata, ascidians and bryozoans on tide-swept sublittoral fringe rock
| Researched by | Thomas Stamp, Charlotte Marshall, Emma Williams, Kelsey Lloyd & Megan Mardle | Refereed by | This information is not refereed |
|---|
Summary
UK and Ireland classification
Description
Very sheltered bedrock, boulders and cobbles that are subject to moderate to strong tidal water movement characterized by dense Laminaria digitata, ascidians and bryozoans. Species richness is generally greater than in the non tide-swept Laminaria digitata biotope (MIR.Ldig.Ldig), with a greater abundance and wider range of foliose red seaweeds. The increased water movement encourages several filter-feeding faunal groups to occur. The sponges Leucosolenia spp., Halichondria panicea and Hymeniacidon perleve frequently occur on steep and overhanging faces. In addition, the ascidians Ascidia conchilega, Dendrodoa grossularia and colonial ascidians are also found. Areas where increased tidal movement influences such a community are in the narrows and/or intertidal sills of sealochs. This biotope may be found immediately below the tide-swept Fucus serratus biotope (SLR.Fserr.T). The sublittoral fringe of similarly sheltered shores that are not tide-swept are generally characterized by mixed Saccharina latissima and Laminaria digitata (SIR.Slat.Ldig) or Saccharina latissima (SIR.Slat). (Information taken from the Marine Biotope Classification for Britain and Ireland, Version 97.06: Connor et al., 1997a, b).
Depth range
Lower shore, 0-5 mAdditional information
-
Listed By
Habitat review
Ecology
Ecological and functional relationships
Kelp habitats are dynamic ecosystems where competition for space, light and food result in patchy distribution patterns of flora and fauna. Kelp beds are diverse species rich habitats and over 1,200 species have been recorded in UK moderately exposed kelp biotopes (MIR.KR) (Birkett et al., 1998b). Kelps are major primary producers; up to 90% of kelp production enters the detrital food web and is probably a major contributor of organic carbon to surrounding communities (Birkett et al., 1998b). Major interactions are thought to be the effects of competition for space, shading, herbivory and predation.- In most kelp biotopes there is evidence of strong competition for space, especially for space on a favourable substratum. Competition may occur between individual plants of the same species, between kelps and substratum-colonizing species of animals and other algae and between colonial animals and encrusting algae. Competition for space between individuals and species is dynamic, resulting in a constantly changing patchwork of species covering any suitable substrata within the biotope, including the surface of the kelp plants themselves. This is especially true of the components of tide-swept biotopes such as MIR.Ldig.T. Tide swept biotopes offer luxuriant conditions for suspension feeders by providing a continual supply of food and removing finer sediment that may otherwise interfere with their delicate feeding apparatus. As a result, strong competition between the suspension feeders that thrive in this biotope will mean that any available substratum is likely to be colonized. Much of the rock surface will be covered by a 'foundation' of encrusting calcareous algae on top of which other species will grow.
- The blades of Laminaria digitata plants form a canopy layer, which may cut off much of the incident irradiance. This restricts the development of species with high light demands so that the understorey of plants becomes dominated by shade tolerant red algae including Corallinaceae, Palmaria palmata, Chondrus crispus and Ceramium nodulosum. It also allows species normally restricted to the lower infralittoral in kelp-free areas to compete more effectively in the reduced light levels of the kelp bed and so are found at shallower depths.
- Within kelp beds there are relatively few species that graze either the kelp or the understorey algae directly, as the enzymes required to directly utilize algae as food are not common. However, the gastropod Steromphala cineraria is frequently found in this biotope and may graze the kelp, foliose red seaweeds and the rock below. The blue-rayed limpet Patella pellucida also grazes on kelp and, when younger, red seaweeds such as Mastocarpus stellatus, which is commonly found in the understorey of this biotope. The edible sea urchin Echinus and green sea urchin Psammechinus milaris also graze on kelp species in addition to prey species such as bryozoans, tunicates and hydroids.
- Predation within kelp beds has not been well studied in the United Kingdom and very little is known of the predator-prey relationships for the many species occurring in kelp beds. The common shore crab Carcinus maenas is probably the largest mobile predator associated with this biotope and preys upon Gibbula cineraria.
- As mentioned previously, tide swept biotopes offer a continual supply of suspended particulate matter that support a thriving suspension feeding community. Suspension feeders in MIR.Ldig.T represent several different phyla.
- Sponges Of the sponges, the breadcrumb sponge Halichondria panicea is most commonly associated with this biotope. This species is usually found as an encrusting mat on rock and algae. Hymeniacidon perleve is also likely to be present.
- Ascidians Both solitary and colonial ascidians are found in this biotope. The colonial ascidians Botryllus schlosseri (the star ascidian) and Botrylloides leachi, and the solitary baked bean sea squirt Dendrodoa grossularia are all frequent.
- Cnidaria Several hydroid species are commonly found on rock below the kelp in this biotope, especially Dynamena pumila and Sertularia argentea.
- Crustacea Crustacean suspension feeders associated with this biotope are not the most important group, in terms of frequency and abundance, but include the barnacles Balanus crenatus and Semibalanus balanoides.
- Annelida The tube worm Spirobranchus triqueter is the most common suspension feeing annelid associated within this biotope. It was found in two thirds of the records of this biotope and can rapidly colonize patches of bare rock. Spirorbid worms may be found.
- Bryozoa Alcyonidium gelatinosum, Alcyonidium hirsutum, Electra pilosa, Membranipora membranacea and Scrupocellaria spp. are all likely to compete for space on the fronds and stipes of the kelp plants.
- Kelp plants are also exploited as a habitat; the holdfast, stipe and frond each support a different type of community, although only the oldest Laminaria digitata plants will have epiphytic flora and fauna on the stipe (which is smooth in all but the oldest individuals). However, holdfasts shelter a particularly rich diversity of animals from a wide range of taxa (see Habitat Complexity). Epiphytes on the stipe may include the sponge Halichondria panicea and red algae Palmaria palmata and Phycodrys rubens whereas the frond is more likely to be colonized by the bryozoan Membranipora membranacea.
Seasonal and longer term change
Present understanding of the natural fluctuations in the species assemblages, populations, distribution and diversity of species in kelp beds is limited. The plants in this biotope are likely to experience some seasonal change in abundance, the general pattern being a lower percentage cover over the winter months. However, this biotope is limited to extremely sheltered habitats and therefore, the occurrence of winter storms is unlikely to affect it to the same extent that more wave exposed habitats would be affected.- Growth rate of Laminaria digitata is seasonally controlled with a period of rapid growth from February to July and one of slower growth from August to January. Increased wave exposure and storms experienced during winter months may erode Laminaria digitata blades and reduce the overall standing biomass. Periodic storms may remove older and weaker plants creating patches cleared of kelp. Cleared patches may encourage growth of sporelings or gametophyte maturation. Growth of understorey algae may also be reduced in the winter months.
- Concomitant with the reduction in available surface area of Laminaria digitata blades, a proportion of epiphytic bryozoans, ascidians and sponges will also be lost. However, epilithic representatives of these species will remain on the bedrock and boulders.
- Increased wave exposure and storm frequency over the winter months may also increase the frequency of impacts from wave driven debris, such as pebbles and boulders. These impacts may create 'bare' patches on the surface of the bedrock, and the boulders themselves, which may be colonized by fast growing species including the tube worm Spirobranchus triqueter.
Habitat structure and complexity
Owing to the tide-swept habitat with which this biotope is associated, a diverse marine life is supported. The fast currents provide a continual supply of suspended material sustaining a profusion of both active and passive suspension feeders that dominate the fauna. Fine sediment is removed by the current and the settlement of material, that could otherwise be detrimental to the suspension feeders, is prevented. It is the complex structure of this habitat and its many different niches that allow such a diverse range of suspension feeders to coexist. Almost every possible substratum including the bedrock, boulders and cobbles, and the holdfast, stipe and blade of the Laminaria digitata itself, is covered with various flora and fauna. In addition to the luxuriant conditions for suspension feeders, Hiscock (1983) lists some the benefits of strong water movement to include the potential for a greater photosynthetic efficiency, thereby possibly increasing the depth penetration of the algae. Increased water movement has been associated with an increase in photosynthesis in several algal species including Fucus serratus and Ascophyllum nodosum (Robbins, 1968, cited in Hiscock, 1983).- Holdfasts provide refuge to a wide variety of animals supporting a diverse fauna that may include polychaetes, small crabs, gastropods, bivalves, and amphipods.
- Kelp fronds are likely to be colonized by encrusting bryozoans (e.g. Membranipora membranacea), ascidians (e.g. Botryllus schlosseri), hydroids (e.g. Dynamena pumila) and sponges (e.g. Halichondria panicea).
- Stipes of Laminaria digitata can support a considerable epiphytic flora, mainly of smaller species (Gayral & Cosson, 1973; Jones et al., 2000).
- The bedrock and boulders offer surfaces for settlement and shelter of species and are colonized by encrusting and foliose red algae but dominated by animals including ascidians, bryozoans, sponges and tubicolous worms.
Productivity
- Kelp plants are major primary producers in shallow rocky marine habitats in Britain and Ireland. Within the euphotic zone, kelps produce nearly 75% of the net carbon fixed and large kelps often produce annually well in excess of a kilogram of carbon per square metre of shore. However, only about 10% of this productivity is directly grazed. Kelps contribute 2-3 times their standing biomass each year as particulate detritus and dissolved organic matter that provides the energy supply for filter feeders and detritivores in and around the kelp bed. Dissolved organic carbon, algal fragments and microbial film organisms are continually removed by the sea, which may enter the food chain of local subtidal ecosystems, or be exported further offshore. The Corallinaceae and foliose red algae, although not as significant as the kelp, also contribute to primary production within this biotope.
- The fast currents associated with this biotope provide a continual supply of suspended material that sustains a diverse suspension feeding community. Suspension feeders including sponges, bryozoans, ascidians and hydroids, represent the dominant fauna in this biotope highlighting the importance of secondary production.
- Rocky shores make a contribution to the food of many marine species through the production of planktonic larvae and propagules which contribute to pelagic food chains.
Recruitment processes
- Laminaria digitata plants are fertile all year round with maximum production of spores in July - August and November - December. Young sporophytes (germlings) appear all year with maxima in spring and autumn. Chapman (1981) demonstrated that substantial recruitment of Laminaria digitata plants to areas barren of kelp plants was possible up to 600 m away from reproductive plants.
- Kelp plants themselves can affect recruitment in other species through their influence on the underlying substrata. Shading and mechanical sweeping, for example, will adversely affect settling larvae and post settlement survival.
- With respect to the underlying red algae, tetrasporangia from Corallina officinalis have been recorded throughout the year although settlement occurs after a couple of days which has the potential to limit dispersal. Recruitment in dulse, Palmaria palmata, is most certainly limited in terms of dispersal. Females do not release carpospores so male gametophytes produce spermatia which sink rapidly to enable the male and females gametes to come into contact for fertilization. Lithophyllum incrustans reproduce annually and it has been calculated that 1 mm² of reproductive thallus produces 17.5 million bispores per year with an average settlement of only 55 sporelings/year (Edyvean & Ford, 1984).
- The majority of characteristic fauna associated with this biotope produce planktonic larvae and therefore, depending on respective plankton durations, recruitment is possible from both local sources and populations further away. Breeding in the bryozoan Membranipora membranacea continues through early summer with planktonic cyphonautes settling proceeding into early autumn (Ryland & Hayward, 1977). Spirobranchus triqueter, a tubeworm, produces planktonic all year around, although settlement appears to be limited in winter months.
Time for community to reach maturity
Kain (1975) examined the recolonization of cleared concrete blocks by kelp plants and other algae and found that Laminaria digitata plants were re-established within 2 years and that red algae returned with a year. Many other characterizing species have planktonic larvae and/or are mobile and so can migrate into the affected area. Colonization of most species of fauna inhabiting kelp holdfast, for example, were found as early as one year after kelp trawling of Laminaria hyperborea plants in Norway, although numbers of both individuals and species, especially isopods and amphipods, increase with a corresponding increase in holdfast size (Christie et al., 1998). However, although these species colonize the biotope quite rapidly maturity of the overall community is likely to be longer (see 'Recoverability'). For example, encrusting coralline algae such as Lithophyllum incrustans are slow growing (2-7 mm per annum - see Irvine & Chamberlain, 1994) and recruitment of other species to the kelp bed may take longer. In dredged kelp beds in Norway for example, although the rock between Laminaria hyperborea plants was uniformly covered with coralline algae after 3 years, the more diverse community of cnidarians, bryozoans and sponges associated with coralline algae seen on undredged plots was absent (Rinde et al., 1992, cited in Birkett et al., 1998). Although it was suggested that the kelp forest recovered to an almost 'normal' state within 3 to 4 years, full biological restoration after harvesting may take at least ten years (Birkett et al., 1998b).Additional information
-Preferences & Distribution
Habitat preferences
| Depth Range | Lower shore, 0-5 m |
|---|---|
| Water clarity preferences | No information |
| Limiting Nutrients | Data deficient |
| Salinity preferences | Full (30-40 psu) |
| Physiographic preferences | No information |
| Biological zone preferences | Sublittoral fringe |
| Substratum/habitat preferences | Bedrock, Cobbles, Large to very large boulders, Small boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Very strong > 6 knots (>3 m/sec.) |
| Wave exposure preferences | Extremely sheltered, Sheltered, Very sheltered |
| Other preferences | Extremely sheltered from wave exposure. |
Additional Information
This biotope is associated with areas of moderate to strong water flow rates. It is typically found in narrow channels, shallow lagoons/rapids or the entrances to fjordic sea lochs and to obs. In the Menai Strait and Loch Roag the biotope experiences tidal flow rates of up to 8 knots (Brazier et al., 1999).
Species composition
Species found especially in this biotope
Rare or scarce species associated with this biotope
-
Additional information
The MNCR recorded 425 species in 45 records of this biotope although not all the species occurred in all records of the biotope (JNCC, 1999).Sensitivity review
Sensitivity characteristics of the habitat and relevant characteristic species
IR.MIR.KT.LdigT occurs on very sheltered bedrock, boulders and cobbles that are subject to moderate to strong tidal steams. The community is characterized by a Laminaria digitata canopy, beneath which is a diverse understorey community of varied red seaweed, ascidians and bryozoans. High tidal flow encourages several filter-feeding faunal groups to occur. The sponges Leucosolenia spp., Halichondria panicea and Hymeniacidon perleve frequently occur on steep and overhanging faces. In addition, the ascidians Ascidia conchilega, Dendrodoa grossularia and colonial ascidians are also found.
In undertaking this assessment of sensitivity, account is taken of knowledge of the biology of all characterizing species in the biotope. For this sensitivity assessment Laminaria digitata is the primary focus of research, however, it is recognized that the tide swept faunal community also define the biotope. Examples of important species groups are mentioned where appropriate.
Resilience and recovery rates of habitat
In general, the available evidence indicates that the recovery of kelp biotopes, where kelp have been entirely removed, requires at least two years to recover. Re-colonization of concrete blocks by Laminaria digitata was investigated by Kain (1975) at Port Erin, Isle of Man. Laminaria digitata was considered re-established two years after removal, with the characterizing red foliose algae following one year later. Similarly, recovery after simulated harvesting of a standing crop of Laminaria digitata occurred within 18-20 months (Kain, 1979). While colonization of young Laminaria sporophytes may occur one year after initial substratum clearance (Kain 1979), the return of the biotope to its original mature condition is likely to lag behind this recolonization. These findings agree with previous studies which showed that when 60% of sporophytes (adult alga) were removed from a location, 18 months were required for the stand to rejuvenate (Perez, 1971), while in France, CIAM (Le Comité interprofessionel des algues marines) proposed that, regardless of collection method, the restoration of stands of Laminarians took up to 18 months post harvesting (Arzel, 1998). Some disparities between reported recovery rates do exist, with cleared plots in Helgoland taking 25 months, probably because plots were burned to ensure total removal of spores and germlings (Markham & Munda, 1980). Even after 25 months, although macroalgal density had returned to pre-clearance levels, the Laminaria digitata were smaller than those on undisturbed plots, suggesting full recovery needs longer than 25 months ( Markham & Munda, 1980).
The seasonal timing of macroalgal removal impacts recovery rates. Engelen et al. (2010) showed that removal of 0.25m2 areas of Laminaria digitata forest in the spring and autumn had different recovery rates, with autumn recovery more rapid than spring (taking a minimum of 12 months). Return to conditions prior to removal took 18-24 months, with competition for space by Saccorhiza polyschides impacting recovery rates in the first year of recolonization (Engelen et al., 2010). The growth rate of Laminaria digitata changes with the seasons. Growth is rapid from February to July, slower in August to January, and occurs diffusely in the blade (Kain, 1979). This diffuse growth may enhance its resistance to potential grazers. Spores are produced at temperatures lower than 18 °C with a minimum of 10 weeks a year between 5-18 °C needed to ensure spore formation (Bartsch, 2013). Thus temperature and by default season impacts the level of reproductive activity. In order to maximise survival rates of mature gametophytes, gametophyte development can be delayed by the algae until optimum conditions return and the gametophyte produces gametes (Hoek van den et al., 1995), which suggests a degree of resistance to short-term changes in temperature that may be anthropogenic in origin. However, seaweeds have been cited as being particularly sensitive to short-term warming events (Dayton & Tegner, 1984; Smale & Wernberg, 2013; Wernberg et al., 2013; from Smale et al., 2013).
Smith (1985) recorded the recovery of Laminaria longicruris and Laminaria digitata following total experimental clearance within Lobster bay, Nova Scotia. Within three months Laminaria longicruris recovery was well established, and experimental clearance plots were indistinguishable from the surrounding habitat. Laminaria digitata however required two years to fully recover following clearance.
Biological traits that influence the recovery rates of Laminaria digitata include its reproductive strategy and life history. The dispersal of Laminaria digitata’s spores and subsequent successful recruitment has been recorded 600 m from reproductive individuals (Chapman, 1981). Local water movement plays an important role in the potential recovery of a biotope, with spores dependent on currents to extend their dispersal range, although the majority of larvae settle within its local habitat (Brennan et al., 2014).
The frequency of disturbance is also important when considering the resilience of this biotope to various pressures, especially in terms of allowing novel species to out-compete Laminaria digitata in local areas. A loss in genetic diversity is not regarded as an issue for this species, unless additional pressures result in the isolation and fragmentation of wild coastal populations (Valero et al., 2011). Genetic differentiation in wild populations occurs within 10 km with genetic flow occurring between adjacent species (Billot et al., 2003). Opportunistic species such as Sargassum muticum and Codium fragile exploit gaps in the kelp bed and out-compete Laminaria digitata, so that high frequency, low impact disturbances may make the kelp stands more vulnerable to competition from these and other turf forming algae; especially if climate change results in temperature shifts (Staehr et al., 2000; Scheibling & Gagnon, 2006; Connell & Russell 2010).
Experimental work in Nova Scotia (Atlantic coast of Canada), where Laminaria longicruris (and its understorey of Laminaria digitata) is harvested has shown that if kelps beds are destroyed or partially destroyed, grazing sea urchins may prevent regeneration and recruitment of kelp populations. It is thought that kelp harvesting removes the cover and protection of urchin predators (lobsters, crabs, fish) and a reduction in predator pressure, due either to kelp harvesting or lobster fishing, enables increases in urchin populations which graze destructively on Laminaria spp. , forming barrens (Bernstein et al. 1981). Grazers are responsible for less than 20% of kelp produced nutrients entering the food web; the majority enters as detritus or dissolved organic matter. Under healthy conditions, grazers do not feed on the kelp themselves, but on their epibiota, with a few rare examples such as the blue-rayed limpet (Krumhansl & Scheibling, 2012). The urchin barrens recorded off the coast of Norway are not common to UK waters with deforestation by urchins instead restricted and patchy (although some have been noted in Scotland; Smale et al., 2013). Stressed environments may be more susceptible to overgrazing by urchins, highlighting the need to consider these stressors as accumulative rather than isolated.
Resilience assessment. Evidence from Engelen et al. (2011) indicated that complete recovery of Laminaria digitata and its associated epibiota occurred 18-24 month after complete removal of Laminaria digitata. Smith (1985) also suggested 24 months for the recovery of a Laminaria digitata bed.
Therefore, resilience has been assessed as ‘High’. Competition between Laminaria digitata and Saccorhiza polyschides can also increase recovery time. In addition, experimental evidence (Kain, 1975, 1979; Markham & Munda, 1980) suggest that if the entire community is removed (e.g. where resistance is 'None') that the recovery of the kelp bed and red algal community may take longer, possibly up to three years, so that resilience is assessed as 'Medium'.
Climate Change Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Global warming (extreme) [Show more]Global warming (extreme)Extreme emission scenario (by the end of this century 2081-2100) benchmark of:
EvidenceThe distribution of kelp is strongly influenced by climatic conditions; therefore, kelp species are extremely sensitive to the ongoing ocean warming (Kain, 1979; Van Den Hoek, 1982; Breeman, 1990; Lüning, 1990; Assis et al., 2016; Smale, 2020). Northern distribution boundaries are set by winter temperatures that are lethal, or summer temperatures too low for growth and/or reproduction, while southern limits are set by high lethal summer temperatures or winter temperatures too high for the induction of a crucial step in the life cycle (Breeman, 1990). Kelps have a high dependence on ocean temperatures, which make them highly vulnerable to ocean warming (Assis et al., 2014). As temperatures increase, populations found towards the upper limit of their temperature range may be adversely affected by warming as physiological thresholds are exceeded (Wiens, 2016). Thermal stress can lead to mortality and consequent population-level effects, such as decreased abundance, altered size structure, local extinction and range contractions (Smale, 2020). Laminaria digitata is a boreal species of kelp, distributed from Brittany to the coast of Norway, while its UK distribution encompasses the whole of the UK coast (Blight & Thompson, 2008). Laminaria digitata distribution suggests that the species would tolerate chronic temperature change (e.g. by 2°C for a year). However, local populations may have acclimatized to local physical conditions meaning that populations at the extremes of the species’ range are less comparable than those populations in the middle of its range. In addition, the distribution of this species suggests that Laminaria digitata is a northern species and, as such, may be vulnerable to increases in temperature and could be outcompeted at its southern limits by other kelp species. Laminaria digitata can grow over a range of temperatures with a thermal optimum between 10-15°C (Bolton & Lüning 1982; Dieck 1992; Arzel, 1998). With optimum reproductivity between 5-10°C, and reproductive ability impaired to 20% at 18°C (Arzel, 1998; Bartsch et al., 2013). Therefore, while the current population may not be affected, recruitment may be reduced. Spore production only occurs between 5-10°C and is the most temperature sensitive stage of reproduction in Laminaria digitata. A minimum of ten weeks a year between 5-18°C is needed in order to ensure spore formation and hence reproduction (Bartsch et al., 2013). Outside this temperature range, reproduction is severely reduced, and the species is at risk from local extinction in the long-term. Studies have shown temperatures of 18-20°C to cause reduced growth (Hargrave et al., 2017), tissue loss (Simonson et al., 2015) and increased mortality (Wilson et al., 2015) of Laminaria digitata. Therefore, the sensitivity of this species relies on the current sea temperatures of the specific location (Bartsch et al., 2013). Merzouk & Johnson (2011) combined predicted sea surface temperate over the next century with the current distribution of Laminaria digitata and predicted an expansion of its northern limits and localised extinctions across its southern range (mid Bay of Biscay, Northern France and southern England; Birkett et al., 1998b). These results suggest that local extinction of the biotope may occur at sites where sea temperature is artificially increased as a result of anthropogenic activity (e.g. effluent output) (Raybaud et al., 2013), especially if combined with high UK summer sea temperatures in southern examples of this biotope (Bartsch et al., 2013). In southern examples of IR.MIR.KT.LdigT, Laminaria digitata may also be out-competed by its Lusitanian competitor Laminaria ochroleuca, which is regionally abundant across the south UK coastline (Smale et al., 2014). Sensitivity assessment. As temperatures rise to up to 20°C, it is predicted that Laminaria digitata could be lost and that more, warm adapted, algae will take its place. Sea surface temperatures around the UK are currently between 6-19°C (Huthnance, 2010). Under the middle emission scenario, Laminaria digitata is likely to be lost in southern parts of the UK, where summer temperatures would exceed 20°C, but is likely to remain present around the rest of the UK. Therefore, resistance is assessed as ‘Low’ under the middle emission scenario. Resilience is assessed as ‘Very Low’, as the loss is likely to be a long-term decline, due to the long-term nature of ocean warming. Therefore, this biotope IR.MIR.KT.LdigT is assessed as having ‘High’ sensitivity to ocean warming under a middle emission scenario. For the high emission scenario and extreme scenario, sea temperatures may rise by 4-5°C to give potential southern summer temperatures of 23-24°C and northern summer temperatures of 18-19°C. Under these scenarios, it is likely that Laminaria digitata will retreat northwards, with large losses across England, Ireland, and Wales. Therefore, resistance is assessed as ‘None’, and resilience is assessed as ‘Very low’. Overall, this biotope is assessed as having ‘High’ sensitivity to ocean warming for the high emission scenario and the extreme scenario. | LowHelp | Very LowHelp | HighHelp |
Global warming (high) [Show more]Global warming (high)High emission scenario (by the end of this century 2081-2100) benchmark of:
EvidenceThe distribution of kelp is strongly influenced by climatic conditions; therefore, kelp species are extremely sensitive to the ongoing ocean warming (Kain, 1979; Van Den Hoek, 1982; Breeman, 1990; Lüning, 1990; Assis et al., 2016; Smale, 2020). Northern distribution boundaries are set by winter temperatures that are lethal, or summer temperatures too low for growth and/or reproduction, while southern limits are set by high lethal summer temperatures or winter temperatures too high for the induction of a crucial step in the life cycle (Breeman, 1990). Kelps have a high dependence on ocean temperatures, which make them highly vulnerable to ocean warming (Assis et al., 2014). As temperatures increase, populations found towards the upper limit of their temperature range may be adversely affected by warming as physiological thresholds are exceeded (Wiens, 2016). Thermal stress can lead to mortality and consequent population-level effects, such as decreased abundance, altered size structure, local extinction and range contractions (Smale, 2020). Laminaria digitata is a boreal species of kelp, distributed from Brittany to the coast of Norway, while its UK distribution encompasses the whole of the UK coast (Blight & Thompson, 2008). Laminaria digitata distribution suggests that the species would tolerate chronic temperature change (e.g. by 2°C for a year). However, local populations may have acclimatized to local physical conditions meaning that populations at the extremes of the species’ range are less comparable than those populations in the middle of its range. In addition, the distribution of this species suggests that Laminaria digitata is a northern species and, as such, may be vulnerable to increases in temperature and could be outcompeted at its southern limits by other kelp species. Laminaria digitata can grow over a range of temperatures with a thermal optimum between 10-15°C (Bolton & Lüning 1982; Dieck 1992; Arzel, 1998). With optimum reproductivity between 5-10°C, and reproductive ability impaired to 20% at 18°C (Arzel, 1998; Bartsch et al., 2013). Therefore, while the current population may not be affected, recruitment may be reduced. Spore production only occurs between 5-10°C and is the most temperature sensitive stage of reproduction in Laminaria digitata. A minimum of ten weeks a year between 5-18°C is needed in order to ensure spore formation and hence reproduction (Bartsch et al., 2013). Outside this temperature range, reproduction is severely reduced, and the species is at risk from local extinction in the long-term. Studies have shown temperatures of 18-20°C to cause reduced growth (Hargrave et al., 2017), tissue loss (Simonson et al., 2015) and increased mortality (Wilson et al., 2015) of Laminaria digitata. Therefore, the sensitivity of this species relies on the current sea temperatures of the specific location (Bartsch et al., 2013). Merzouk & Johnson (2011) combined predicted sea surface temperate over the next century with the current distribution of Laminaria digitata and predicted an expansion of its northern limits and localised extinctions across its southern range (mid Bay of Biscay, Northern France and southern England; Birkett et al., 1998b). These results suggest that local extinction of the biotope may occur at sites where sea temperature is artificially increased as a result of anthropogenic activity (e.g. effluent output) (Raybaud et al., 2013), especially if combined with high UK summer sea temperatures in southern examples of this biotope (Bartsch et al., 2013). In southern examples of IR.MIR.KT.LdigT, Laminaria digitata may also be out-competed by its Lusitanian competitor Laminaria ochroleuca, which is regionally abundant across the south UK coastline (Smale et al., 2014). Sensitivity assessment. As temperatures rise to up to 20°C, it is predicted that Laminaria digitata could be lost and that more, warm adapted, algae will take its place. Sea surface temperatures around the UK are currently between 6-19°C (Huthnance, 2010). Under the middle emission scenario, Laminaria digitata is likely to be lost in southern parts of the UK, where summer temperatures would exceed 20°C, but is likely to remain present around the rest of the UK. Therefore, resistance is assessed as ‘Low’ under the middle emission scenario. Resilience is assessed as ‘Very Low’, as the loss is likely to be a long-term decline, due to the long-term nature of ocean warming. Therefore, this biotope IR.MIR.KT.LdigT is assessed as having ‘High’ sensitivity to ocean warming under a middle emission scenario. For the high emission scenario and extreme scenario, sea temperatures may rise by 4-5°C to give potential southern summer temperatures of 23-24°C and northern summer temperatures of 18-19°C. Under these scenarios, it is likely that Laminaria digitata will retreat northwards, with large losses across England, Ireland, and Wales. Therefore, resistance is assessed as ‘None’, and resilience is assessed as ‘Very low’. Overall, this biotope is assessed as having ‘High’ sensitivity to ocean warming for the high emission scenario and the extreme scenario. | LowHelp | Very LowHelp | HighHelp |
Global warming (middle) [Show more]Global warming (middle)Middle emission scenario (by the end of this century 2081-2100) benchmark of:
EvidenceThe distribution of kelp is strongly influenced by climatic conditions; therefore, kelp species are extremely sensitive to the ongoing ocean warming (Kain, 1979; Van Den Hoek, 1982; Breeman, 1990; Lüning, 1990; Assis et al., 2016; Smale, 2020). Northern distribution boundaries are set by winter temperatures that are lethal, or summer temperatures too low for growth and/or reproduction, while southern limits are set by high lethal summer temperatures or winter temperatures too high for the induction of a crucial step in the life cycle (Breeman, 1990). Kelps have a high dependence on ocean temperatures, which make them highly vulnerable to ocean warming (Assis et al., 2014). As temperatures increase, populations found towards the upper limit of their temperature range may be adversely affected by warming as physiological thresholds are exceeded (Wiens, 2016). Thermal stress can lead to mortality and consequent population-level effects, such as decreased abundance, altered size structure, local extinction and range contractions (Smale, 2020). Laminaria digitata is a boreal species of kelp, distributed from Brittany to the coast of Norway, while its UK distribution encompasses the whole of the UK coast (Blight & Thompson, 2008). Laminaria digitata distribution suggests that the species would tolerate chronic temperature change (e.g. by 2°C for a year). However, local populations may have acclimatized to local physical conditions meaning that populations at the extremes of the species’ range are less comparable than those populations in the middle of its range. In addition, the distribution of this species suggests that Laminaria digitata is a northern species and, as such, may be vulnerable to increases in temperature and could be outcompeted at its southern limits by other kelp species. Laminaria digitata can grow over a range of temperatures with a thermal optimum between 10-15°C (Bolton & Lüning 1982; Dieck 1992; Arzel, 1998). With optimum reproductivity between 5-10°C, and reproductive ability impaired to 20% at 18°C (Arzel, 1998; Bartsch et al., 2013). Therefore, while the current population may not be affected, recruitment may be reduced. Spore production only occurs between 5-10°C and is the most temperature sensitive stage of reproduction in Laminaria digitata. A minimum of ten weeks a year between 5-18°C is needed in order to ensure spore formation and hence reproduction (Bartsch et al., 2013). Outside this temperature range, reproduction is severely reduced, and the species is at risk from local extinction in the long-term. Studies have shown temperatures of 18-20°C to cause reduced growth (Hargrave et al., 2017), tissue loss (Simonson et al., 2015) and increased mortality (Wilson et al., 2015) of Laminaria digitata. Therefore, the sensitivity of this species relies on the current sea temperatures of the specific location (Bartsch et al., 2013). Merzouk & Johnson (2011) combined predicted sea surface temperate over the next century with the current distribution of Laminaria digitata and predicted an expansion of its northern limits and localised extinctions across its southern range (mid Bay of Biscay, Northern France and southern England; Birkett et al., 1998b). These results suggest that local extinction of the biotope may occur at sites where sea temperature is artificially increased as a result of anthropogenic activity (e.g. effluent output) (Raybaud et al., 2013), especially if combined with high UK summer sea temperatures in southern examples of this biotope (Bartsch et al., 2013). In southern examples of IR.MIR.KT.LdigT, Laminaria digitata may also be out-competed by its Lusitanian competitor Laminaria ochroleuca, which is regionally abundant across the south UK coastline (Smale et al., 2014). Sensitivity assessment. As temperatures rise to up to 20°C, it is predicted that Laminaria digitata could be lost and that more, warm adapted, algae will take its place. Sea surface temperatures around the UK are currently between 6-19°C (Huthnance, 2010). Under the middle emission scenario, Laminaria digitata is likely to be lost in southern parts of the UK, where summer temperatures would exceed 20°C, but is likely to remain present around the rest of the UK. Therefore, resistance is assessed as ‘Low’ under the middle emission scenario. Resilience is assessed as ‘Very Low’, as the loss is likely to be a long-term decline, due to the long-term nature of ocean warming. Therefore, this biotope IR.MIR.KT.LdigT is assessed as having ‘High’ sensitivity to ocean warming under a middle emission scenario. For the high emission scenario and extreme scenario, sea temperatures may rise by 4-5°C to give potential southern summer temperatures of 23-24°C and northern summer temperatures of 18-19°C. Under these scenarios, it is likely that Laminaria digitata will retreat northwards, with large losses across England, Ireland, and Wales. Therefore, resistance is assessed as ‘None’, and resilience is assessed as ‘Very low’. Overall, this biotope is assessed as having ‘High’ sensitivity to ocean warming for the high emission scenario and the extreme scenario. | MediumHelp | Very LowHelp | MediumHelp |
Marine heatwaves (high) [Show more]Marine heatwaves (high)High emission scenario benchmark: A marine heatwave occurring every two years, with a mean duration of 120 days, and a maximum intensity of 3.5°C. Further detail. EvidenceMarine heatwaves are extreme weather events defined as periods of extreme sea surface temperature that persists for days to months (Frölicher et al., 2018). Marine heatwaves are predicted to occur more frequently, last for longer and at increased intensity by the end of this century under both middle and high emission scenarios (Frölicher et al., 2018). Marine heatwaves are known to cause significant impacts to kelp forests, particularly if a population is found towards the edge of its southern limit (Smale et al., 2019). Changes in primary productivity, community composition, and biogeography have been associated with marine heatwaves (Smale et al., 2019). In Baja California, Mexico, an extreme heat even between 2014– 2016, led to both a decrease in density of Macrocystis pyrifera and a decrease in the number of fronds per individual in Baja California, Mexico (Arafeh-Dalmau et al., 2019). In addition, there was a significant change to the understorey algal composition, and half of the fish and invertebrates associated with this habitat disappeared. The same heatwave, coupled with a loss of starfish through disease and an increase in urchin grazing, led to the loss of >90% of Macrocystis pyrifera from 350 km of coastline in northern California (Rogers-Bennett & Catton, 2019). In Western Australia, marine heatwaves have extremely affected marine ecosystems with widespread loss of Ecklonia radiata, which has changed biotope structure with many kelp forests being replaced by turf algae (Filbee- Dexter & Wernberg, 2018). Similarly, marine heatwaves have also led to the local extinctions of bull kelp Durvillaea antarctica from the coastlines of New Zealand, which then provided space for the invasive kelp Undaria pinnatifida to colonise (Thomsen et al., 2019). Under experimental conditions, Nepper-Davidson et al. (2019) exposed a northern (Denmark) population of Saccharina lattisima to a simulated three-week heatwave of three different intensities; 18, 21 and 24°C. When exposed to heatwaves of 18 and 21°C there was a decrease in photosynthesis and growth. When 24°C was simulated, 91% of sporophytes were dead within a week, and the fronds of the few survivors were disintegrating, so the experiment was terminated (Nepper-Davidsen et al., 2019). Sensitivity assessment. Under the middle emission scenario, if heatwaves occurred every three years, with a maximum intensity of 2°C for 80 days by the end of this century, this could lead to summer sea temperatures reaching up to 24°C in southern England and 19°C in Scotland. Under the middle emission scenario, Laminaria digitata is likely to be lost from the southern parts of the UK (see Global warming pressure). In Scotland, a significant proportion of Laminaria digitata is likely to survive in areas where temperatures do not exceed 20°C but will suffer mortality elsewhere. Therefore, resistance has been assessed as ‘None’. As a further heatwave is likely to affect this habitat before full recovery, resilience has been assessed as ‘Low.’ Therefore, this biotope is assessed as having ‘High’ sensitivity to marine heatwaves under the middle emission scenario. Under the high emission scenario, if heatwaves occur every two years by the end of this century, reaching a maximum intensity of 3.5°C for 120 days, this could lead to the heatwave lasting the entire summer with temperatures reaching up to 26.5°C in southern England 21.5°C in Scotland. Laminaria digitata may be lost from large parts of the UK as its range contracts (see Global warming pressure). Therefore, resistance has been assessed as ‘None’. As widespread mortality may lead to a lack of viable sporophytes for recruitment, resilience has been assessed as ‘Very low.’ Therefore, this biotope is assessed as having ‘High’ sensitivity to marine heatwaves under the high emission scenario. | NoneHelp | Very LowHelp | HighHelp |
Marine heatwaves (middle) [Show more]Marine heatwaves (middle)Middle emission scenario benchmark: A marine heatwave occurring every three years, with a mean duration of 80 days, with a maximum intensity of 2°C. Further detail. EvidenceMarine heatwaves are extreme weather events defined as periods of extreme sea surface temperature that persists for days to months (Frölicher et al., 2018). Marine heatwaves are predicted to occur more frequently, last for longer and at increased intensity by the end of this century under both middle and high emission scenarios (Frölicher et al., 2018). Marine heatwaves are known to cause significant impacts to kelp forests, particularly if a population is found towards the edge of its southern limit (Smale et al., 2019). Changes in primary productivity, community composition, and biogeography have been associated with marine heatwaves (Smale et al., 2019). In Baja California, Mexico, an extreme heat even between 2014– 2016, led to both a decrease in density of Macrocystis pyrifera and a decrease in the number of fronds per individual in Baja California, Mexico (Arafeh-Dalmau et al., 2019). In addition, there was a significant change to the understorey algal composition, and half of the fish and invertebrates associated with this habitat disappeared. The same heatwave, coupled with a loss of starfish through disease and an increase in urchin grazing, led to the loss of >90% of Macrocystis pyrifera from 350 km of coastline in northern California (Rogers-Bennett & Catton, 2019). In Western Australia, marine heatwaves have extremely affected marine ecosystems with widespread loss of Ecklonia radiata, which has changed biotope structure with many kelp forests being replaced by turf algae (Filbee- Dexter & Wernberg, 2018). Similarly, marine heatwaves have also led to the local extinctions of bull kelp Durvillaea antarctica from the coastlines of New Zealand, which then provided space for the invasive kelp Undaria pinnatifida to colonise (Thomsen et al., 2019). Under experimental conditions, Nepper-Davidson et al. (2019) exposed a northern (Denmark) population of Saccharina lattisima to a simulated three-week heatwave of three different intensities; 18, 21 and 24°C. When exposed to heatwaves of 18 and 21°C there was a decrease in photosynthesis and growth. When 24°C was simulated, 91% of sporophytes were dead within a week, and the fronds of the few survivors were disintegrating, so the experiment was terminated (Nepper-Davidsen et al., 2019). Sensitivity assessment. Under the middle emission scenario, if heatwaves occurred every three years, with a maximum intensity of 2°C for 80 days by the end of this century, this could lead to summer sea temperatures reaching up to 24°C in southern England and 19°C in Scotland. Under the middle emission scenario, Laminaria digitata is likely to be lost from the southern parts of the UK (see Global warming pressure). In Scotland, a significant proportion of Laminaria digitata is likely to survive in areas where temperatures do not exceed 20°C but will suffer mortality elsewhere. Therefore, resistance has been assessed as ‘None’. As a further heatwave is likely to affect this habitat before full recovery, resilience has been assessed as ‘Low.’ Therefore, this biotope is assessed as having ‘High’ sensitivity to marine heatwaves under the middle emission scenario. Under the high emission scenario, if heatwaves occur every two years by the end of this century, reaching a maximum intensity of 3.5°C for 120 days, this could lead to the heatwave lasting the entire summer with temperatures reaching up to 26.5°C in southern England 21.5°C in Scotland. Laminaria digitata may be lost from large parts of the UK as its range contracts (see Global warming pressure). Therefore, resistance has been assessed as ‘None’. As widespread mortality may lead to a lack of viable sporophytes for recruitment, resilience has been assessed as ‘Very low.’ Therefore, this biotope is assessed as having ‘High’ sensitivity to marine heatwaves under the high emission scenario. | NoneHelp | LowHelp | HighHelp |
Ocean acidification (high) [Show more]Ocean acidification (high)High emission scenario benchmark: a further decrease in pH of 0.35 (annual mean) and corresponding 120% increase in H+ ions , seasonal aragonite saturation of 20% of UK coastal waters and North Sea bottom waters, and the aragonite saturation horizon in the NE Atlantic, off the continental shelf, occurring at a depth of 400 m by the end of this century 2081-2100. Further detail EvidenceIncreasing levels of CO2 in the atmosphere have led to the average pH of sea surface waters dropping from 8.25 in the 1700s to 8.14 in the 1990s (Jacobson, 2005), with it expected to drop up to a further 0.35 units by the end of this century, dependent on the emission scenario. Marine autotrophs will generally benefit from ocean acidification, through an increase in the availability of aqueous CO2 for photosynthesis (Koch et al., 2013). Most species of kelp appear to be under-saturated in respect to carbon dioxide, although they can generally utilise HCO3 and have external carbonic anhydrase for extracellular dehydration of HCO3 to CO2 (Koch et al., 2013). Under experimental conditions, Iñiguez et al. (2016a) found that although photosynthesis remained stable in Alaria esculenta in response to increasing CO2, the growth rate increased. Similarly, Gordillo et al. (2015) found heightened growth rates in Alaria esculenta when exposed to increased CO2, although this increase was not significant and less pronounced than in Saccharina lattisima. Research on other kelp species has revealed a positive or neutral effect of ocean acidification (Roleda et al., 2012, Fernández et al., 2015, Nunes et al., 2015, Iñiguez et al., 2016b, a), except for one study, which found that ocean acidification negatively impacted photosynthesis and growth in the southern hemisphere species, Ecklonia radiata (Britton et al., 2016). While no direct evidence on the impact of ocean acidification on Laminaria digitata was found, it is likely that this species will either benefit or not be negatively impacted by ocean acidification. Sensitivity assessment. Kelp forests occur in a naturally variable pH habitat, with diel fluctuations of 0.3 - 0.45 pH units (Krause-Jensen et al., 2015, Britton et al., 2016), and boundary layer pH fluctuation of up to 0.8 units (Krause-Jensen et al., 2015). Laminaria digitata is not predicted to suffer negative impacts from future acidification. Therefore, under both the middle and high emission scenario resistance is assessed as ‘High’, and resilience is assessed as ‘High’ leading to a score of ‘Not sensitive’. | HighHelp | HighHelp | Not sensitiveHelp |
Ocean acidification (middle) [Show more]Ocean acidification (middle)Middle emission scenario benchmark: a further decrease in pH of 0.15 (annual mean) and corresponding 35% increase in H+ ions with no coastal aragonite undersaturation and the aragonite saturation horizon in the NE Atlantic, off the continental shelf, at a depth of 800 m by the end of this century 2081-2100. Further detail. EvidenceIncreasing levels of CO2 in the atmosphere have led to the average pH of sea surface waters dropping from 8.25 in the 1700s to 8.14 in the 1990s (Jacobson, 2005), with it expected to drop up to a further 0.35 units by the end of this century, dependent on the emission scenario. Marine autotrophs will generally benefit from ocean acidification, through an increase in the availability of aqueous CO2 for photosynthesis (Koch et al., 2013). Most species of kelp appear to be under-saturated in respect to carbon dioxide, although they can generally utilise HCO3 and have external carbonic anhydrase for extracellular dehydration of HCO3 to CO2 (Koch et al., 2013). Under experimental conditions, Iñiguez et al. (2016a) found that although photosynthesis remained stable in Alaria esculenta in response to increasing CO2, the growth rate increased. Similarly, Gordillo et al. (2015) found heightened growth rates in Alaria esculenta when exposed to increased CO2, although this increase was not significant and less pronounced than in Saccharina lattisima. Research on other kelp species has revealed a positive or neutral effect of ocean acidification (Roleda et al., 2012, Fernández et al., 2015, Nunes et al., 2015, Iñiguez et al., 2016b, a), except for one study, which found that ocean acidification negatively impacted photosynthesis and growth in the southern hemisphere species, Ecklonia radiata (Britton et al., 2016). While no direct evidence on the impact of ocean acidification on Laminaria digitata was found, it is likely that this species will either benefit or not be negatively impacted by ocean acidification. Sensitivity assessment. Kelp forests occur in a naturally variable pH habitat, with diel fluctuations of 0.3 - 0.45 pH units (Krause-Jensen et al., 2015, Britton et al., 2016), and boundary layer pH fluctuation of up to 0.8 units (Krause-Jensen et al., 2015). Laminaria digitata is not predicted to suffer negative impacts from future acidification. Therefore, under both the middle and high emission scenario resistance is assessed as ‘High’, and resilience is assessed as ‘High’ leading to a score of ‘Not sensitive’. | HighHelp | HighHelp | Not sensitiveHelp |
Sea level rise (extreme) [Show more]Sea level rise (extreme)Extreme scenario benchmark: a 107 cm rise in average UK by the end of this century (2018-2100). Further detail. EvidenceSea-level rise is occurring through a combination of thermal expansion and ice melt. Sea levels have risen 1-3 mm/yr in the last century (Cazenave & Nerem, 2004, Church et al., 2004, Church & White, 2006). Sea-level rise is expected to lead to substantial loss of intertidal habitats. Rocky shores backed by cliffs constitute about 80% of oceanic coastlines globally and in Britain, 42% of the coastline is hard rock, with many areas having cliffs behind the shore (Jackson & McIlvenny, 2011). Light availability and water turbidity are principal factors in determining kelp depth range (Birkett et al., 1998b), with laminarians being reported to be able to withstand light levels of up to 1% surface irradiance. An increase in depth due to sea-level rise is likely to impact both Laminaria digitata and any understory algae, negatively impacting this biotope. Understanding how sea-level rise will affect exposure and tidal energy is fraught with uncertainty, although evidence appears to suggest that any alterations will be non-linear (Pickering et al., 2012, Li et al., 2016). Modelling potential outcomes of sea-level rise on the tidal and residual currents in the Bohai Sea, China showed effects were site-dependent, with energy either increasing or decreasing (Li et al., 2016). Similarly, Pickering et al. (2012) found a similar pattern around the UK for tidal amplitude. Sensitivity assessment. This biotope IR.MIR.KT.LdigT is recorded from the lower shore to 5 m in depth. As wave surge diminishes with increased depth, sea-level rise is likely to lead to the density of faunal turf reducing at the deeper reaches of this biotope and transitioning into a biotope characterised by kelp and dense red seaweeds. Tidal streams are also likely to be reduced so that LdigT may transition into Ldig with a lower faunal diversity). This biotope LdigT may be able to expand its range and migrate landwards to compensate for sea-level rise, if not constrained by lack of suitable substratum or human modified shorelines. There is likely to be considerable variation between sites, the relative contribution of wave surge and exposure to habitat suitability, and the depth range occupied by the biotope. Hence, it is difficult to assess the effect of the different sea-level rise scenarios. However, as the biotope can occur from 0-20 m in depth, it is assumed at a sea-level rise of 0.5 m, or 0.7 m (middle to high emission scenarios) would have limited effect but that a 1.07 m rise (the extreme emission scenario) might result in loss of some of the deeper extent of the biotope in some sites. Therefore, resistance is assessed as ‘High’ under the middle and high emission scenarios so that resilience is ‘High’ and sensitivity assessed as ‘Not sensitive’. But resistance is possibly ‘Medium’ under the extreme emission scenario so that resilience is ‘Very low’ and sensitivity assessed as ‘Medium’, albeit with ‘Low’ confidence. | MediumHelp | Very LowHelp | MediumHelp |
Sea level rise (high) [Show more]Sea level rise (high)High emission scenario benchmark: a 70 cm rise in average UK by the end of this century (2018-2100). Further detail. EvidenceSea-level rise is occurring through a combination of thermal expansion and ice melt. Sea levels have risen 1-3 mm/yr in the last century (Cazenave & Nerem, 2004, Church et al., 2004, Church & White, 2006). Sea-level rise is expected to lead to substantial loss of intertidal habitats. Rocky shores backed by cliffs constitute about 80% of oceanic coastlines globally and in Britain, 42% of the coastline is hard rock, with many areas having cliffs behind the shore (Jackson & McIlvenny, 2011). Light availability and water turbidity are principal factors in determining kelp depth range (Birkett et al., 1998b), with laminarians being reported to be able to withstand light levels of up to 1% surface irradiance. An increase in depth due to sea-level rise is likely to impact both Laminaria digitata and any understory algae, negatively impacting this biotope. Understanding how sea-level rise will affect exposure and tidal energy is fraught with uncertainty, although evidence appears to suggest that any alterations will be non-linear (Pickering et al., 2012, Li et al., 2016). Modelling potential outcomes of sea-level rise on the tidal and residual currents in the Bohai Sea, China showed effects were site-dependent, with energy either increasing or decreasing (Li et al., 2016). Similarly, Pickering et al. (2012) found a similar pattern around the UK for tidal amplitude. Sensitivity assessment. This biotope IR.MIR.KT.LdigT is recorded from the lower shore to 5 m in depth. As wave surge diminishes with increased depth, sea-level rise is likely to lead to the density of faunal turf reducing at the deeper reaches of this biotope and transitioning into a biotope characterised by kelp and dense red seaweeds. Tidal streams are also likely to be reduced so that LdigT may transition into Ldig with a lower faunal diversity). This biotope LdigT may be able to expand its range and migrate landwards to compensate for sea-level rise, if not constrained by lack of suitable substratum or human modified shorelines. There is likely to be considerable variation between sites, the relative contribution of wave surge and exposure to habitat suitability, and the depth range occupied by the biotope. Hence, it is difficult to assess the effect of the different sea-level rise scenarios. However, as the biotope can occur from 0-20 m in depth, it is assumed at a sea-level rise of 0.5 m, or 0.7 m (middle to high emission scenarios) would have limited effect but that a 1.07 m rise (the extreme emission scenario) might result in loss of some of the deeper extent of the biotope in some sites. Therefore, resistance is assessed as ‘High’ under the middle and high emission scenarios so that resilience is ‘High’ and sensitivity assessed as ‘Not sensitive’. But resistance is possibly ‘Medium’ under the extreme emission scenario so that resilience is ‘Very low’ and sensitivity assessed as ‘Medium’, albeit with ‘Low’ confidence. | HighHelp | HighHelp | Not sensitiveHelp |
Sea level rise (middle) [Show more]Sea level rise (middle)Middle emission scenario benchmark: a 50 cm rise in average UK sea-level rise by the end of this century (2081-2100). Further detail. EvidenceSea-level rise is occurring through a combination of thermal expansion and ice melt. Sea levels have risen 1-3 mm/yr in the last century (Cazenave & Nerem, 2004, Church et al., 2004, Church & White, 2006). Sea-level rise is expected to lead to substantial loss of intertidal habitats. Rocky shores backed by cliffs constitute about 80% of oceanic coastlines globally and in Britain, 42% of the coastline is hard rock, with many areas having cliffs behind the shore (Jackson & McIlvenny, 2011). Light availability and water turbidity are principal factors in determining kelp depth range (Birkett et al., 1998b), with laminarians being reported to be able to withstand light levels of up to 1% surface irradiance. An increase in depth due to sea-level rise is likely to impact both Laminaria digitata and any understory algae, negatively impacting this biotope. Understanding how sea-level rise will affect exposure and tidal energy is fraught with uncertainty, although evidence appears to suggest that any alterations will be non-linear (Pickering et al., 2012, Li et al., 2016). Modelling potential outcomes of sea-level rise on the tidal and residual currents in the Bohai Sea, China showed effects were site-dependent, with energy either increasing or decreasing (Li et al., 2016). Similarly, Pickering et al. (2012) found a similar pattern around the UK for tidal amplitude. Sensitivity assessment. This biotope IR.MIR.KT.LdigT is recorded from the lower shore to 5 m in depth. As wave surge diminishes with increased depth, sea-level rise is likely to lead to the density of faunal turf reducing at the deeper reaches of this biotope and transitioning into a biotope characterised by kelp and dense red seaweeds. Tidal streams are also likely to be reduced so that LdigT may transition into Ldig with a lower faunal diversity). This biotope LdigT may be able to expand its range and migrate landwards to compensate for sea-level rise, if not constrained by lack of suitable substratum or human modified shorelines. There is likely to be considerable variation between sites, the relative contribution of wave surge and exposure to habitat suitability, and the depth range occupied by the biotope. Hence, it is difficult to assess the effect of the different sea-level rise scenarios. However, as the biotope can occur from 0-20 m in depth, it is assumed at a sea-level rise of 0.5 m, or 0.7 m (middle to high emission scenarios) would have limited effect but that a 1.07 m rise (the extreme emission scenario) might result in loss of some of the deeper extent of the biotope in some sites. Therefore, resistance is assessed as ‘High’ under the middle and high emission scenarios so that resilience is ‘High’ and sensitivity assessed as ‘Not sensitive’. But resistance is possibly ‘Medium’ under the extreme emission scenario so that resilience is ‘Very low’ and sensitivity assessed as ‘Medium’, albeit with ‘Low’ confidence. | HighHelp | HighHelp | Not sensitiveHelp |
Hydrological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Temperature increase (local) [Show more]Temperature increase (local)Benchmark. A 5°C increase in temperature for one month, or 2°C for one year. Further detail EvidenceLaminaria digitata is distributed from Brittany to the Spitzbergen (Birkett et al., 1998; Blight & Thompson, 2008). The Northern/Boreal distribution of Laminaria digitata suggests it may be slightly vulnerable to temperature increases in southern examples of IR.MIR.KT.LdigT. The thermal optimum of Laminaria digitata is between 10-15°C, with reproductive ability impaired to 20% at 18°C (Arzel, 1998). Spore production only occurs between 5-10°C and is the most temperature sensitive stage of reproduction in Laminaria digitata. Outside this temperature range, reproduction is severely reduced and the species is at risk from local extinction in the short-term. A temperature increase to 22-23 °C causes cell damage and death (Sudene, 1964; Bolton & Lüning, 1982). During an exceptionally warm summer in Norway Sundene (1964) reported the destruction of Laminaria digitata plants exposed to temperatures of 22-23 °C. The sensitivity of this species therefore relies on the current sea temperatures of the specific location (Bartsch, 2013). A minimum of 10 weeks a year between 5-18 °C is needed in order to ensure spore formation and hence reproduction and recruitment (Bartsch, 2013). Combining predicted sea surface temperate over the next century with the current distribution of Laminaria digitata, Merzouk & Johnson (2011) predict an expansion of it’s northern limits and localised extinctions across it’s southern range edge (Mid Bay of Biscay, Northern France and southern England; Birkett et al, 1998). Suggesting at sites where sea temperature is artificially increased as a result of anthropogenic activity (e.g. effluent output) local extinction of the biotope may occur (Raybaud et al., 2013) especially if combined with high summer sea temperature (Bartsch et al. 2013). In southern examples of IR.MIR.KT.LdigT, Laminaria digitata may also be out-competed by it’s Lusitanian competitor Laminaria ochroleuca which is regionally abundant across the south UK coastline (Smale et al., 2014). The star ascidian Botryllus schlosseri and the breadcrumb sponge Halichondria panicea have large geographical ranges in which the UK is almost central. At the benchmark level these species are therefore likely to be tolerant of chronic temperature changes. IR.MIR.KT.LdigT is distributed throughout the UK (Connor et al., 2004). Northern to southern Sea Surface Temperature (SST) ranges from 8-16°C in summer and 6-13°C in winter (Beszczynska-Möller & Dye, 2013) Sensitivity assessment. Northern examples of this biotope are unlikely to be affected at the benchmark level, however biotopes within the south of the UK where high summer temperatures combined with an increase of 2 & 5 °C would be above the temperature optimum of Laminaria digitata and may therefore cause declines in growth and abundance. Resistance has been assessed as ‘Medium’, Resilience as ‘High’. Sensitivity has been assessed as ‘Low’. | MediumHelp | HighHelp | LowHelp |
Temperature decrease (local) [Show more]Temperature decrease (local)Benchmark. A 5°C decrease in temperature for one month, or 2°C for one year. Further detail EvidenceLaminaria digitata is distributed from Brittany to the Spitzbergen (Birkett et al., 1998; Blight & Thompson, 2008). The Northern/Boreal distribution of Laminaria digitata suggests it would tolerate a decrease in temperature at the benchmark level. Sensitivity assessment. Resistance has been assessed as ‘High’, resilience as ‘High’ and sensitivity as ‘Not sensitive’. | HighHelp | HighHelp | Not sensitiveHelp |
Salinity increase (local) [Show more]Salinity increase (local)Benchmark. A increase in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidenceKelps are tolerant to short-term daily fluctuation in salinity; however they are much less tolerant to long-term changes with growth rates declining typically either side of 20-45 psu (Karsten, 2007). Laminaria digitata tolerates a large salinity range (5-60 psu; Karsen, 2007) at the extremes of this range; decreases in photosynthetic rates were evident, (Gordillo, 2002). Laminaria digitata is considered to be a stenohaline species, therefore this biotope is only found in conditions of full salinity (Connor et al 1997, Connor et al 2004) Axelsson & Axelsson (1987) indicated damage of the plants’ plasma membranes occurs when salinity is below 20 or above 50 psu. Sensitivity assessment. Laminaria digitata is unlikely to tolerate an increase to >40‰ for a year. Resistance to this pressure is considered ‘Low’, and resilience as ‘High’. This biotope is considered ‘Low’ to this pressure. | LowHelp | HighHelp | LowHelp |
Salinity decrease (local) [Show more]Salinity decrease (local)Benchmark. A decrease in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidenceBirkett et al. (1998) suggested that kelps are stenohaline, in that they do not tolerate wide fluctuations in salinity. Growth rate may be adversely affected if the kelp plant is subjected to periodic salinity stress. The lower salinity limit for Laminaria digitata lies between 10 and 15 psu. On the Norwegian coast, Sundene (1964) found healthy Laminaria digitata plants growing between 15 and 25 psu. Axelsson & Axelsson (1987) indicated damage of the plants’ plasma membranes occurs when salinity is below 20 or above 50 psu. Localized, long-term reductions in salinity, to below 20 psu, may result in the loss of kelp beds in affected areas (Birkett et al., 1998). In laboratory experiments maximum rates of photosynthesis and respiration in Palmaria palmata were observed at a salinity 32 psu (Robbins, 1978) although photosynthetic rates were high down to a salinity of 21 psu. Palmaria palmata is likely to be tolerant of small changes in salinity because as an intertidal species it is regularly exposed to precipitation. Corallina officinalis inhabits rock pools and gullies from mid to low water. Therefore, it is likely to be exposed to short-term hyposaline (freshwater runoff and rainfall) and hypersaline (evaporation) events. However, its distribution in the Baltic is restricted to increasingly deep water as the surface salinity decreases, suggesting that it requires full salinity in the long-term (Kinne, 1971). Some of the fauna, including Halichondria panicea are tolerant of wide variety of salinity habitats from reduced to full salinity and are therefore unlikely to be affected by a drop in salinity at the benchmark level. Sensitivity assessment. The evidence suggests that a decrease in one MNCR salinity scale from ‘Full Salinity’ (30-40 psu) to ‘Reduced Salinity’ (18-30 psu) would still be within Laminaria digtata’s salinity tolerance. Furthermore IR.MIR.KT.LdigT is recorded within low salinity (albeit at low occurrence), indicating many of the characterizing species can tolerate <30‰. Resistance has been assessed as ‘High’ and resilience as ‘High’. Therefore, sensitivity of this biotope to a decrease in salinity has been assessed as ‘Not Sensitive’. | HighHelp | HighHelp | Not sensitiveHelp |
Water flow (tidal current) changes (local) [Show more]Water flow (tidal current) changes (local)Benchmark. A change in peak mean spring bed flow velocity of between 0.1 m/s to 0.2 m/s for more than one year. Further detail EvidenceIR.MIR.KT.LdigT is recorded from very strong-very weak tidal streams (Negligible->3 m/s) (Connor et al., 2004). The filter feeding community within understorey community is likely dependent upon high water flow. However, the distribution of IR.MIR.KT.LdigT across a wide range of tidal streams indicates a change in water flow from 0.1-0.2 m/s would not significantly affect IR.MIR.KT.LdigT. Sensitivity assessment. Resistance has been assessed as ‘High’, resilience as ‘High’. Sensitivity has been assessed as ‘Not Sensitive’ at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Emergence regime changes [Show more]Emergence regime changesBenchmark. 1) A change in the time covered or not covered by the sea for a period of ≥1 year or 2) an increase in relative sea level or decrease in high water level for ≥1 year. Further detail EvidenceLaminaria digitata biotopes are predominantly sublittoral but extend into the lower eulittoral and therefore have some ability to resist desiccation. At the sub-littoral fringe Laminaria digitata regularly becomes exposed to air at low water. Dring & Brown (1982) found that plants that lost up 40-50% of their initial water content were still able to return to their original photosynthetic rate on re-immersion. Many species living beneath the kelp canopy, such as Halichondria panicea and Botryllus schlosseri are also found further up the shore and are therefore likely to be tolerant to a certain degree of desiccation. Furthermore, the kelp canopy is likely to protect the algal understorey and benthic fauna from the worst effects of desiccation by the kelp canopy. However, at the benchmark level, some Laminaria digitata plants at the upper extent of the biotope may perish from the effects of desiccation. In turn, flora and fauna in the understorey may die since the canopy offers protection from desiccation, wind and insolation. The upper extent of the biotope may be reduced although this may be counteracted by an extension of the biotope at the lower limit. Sensitivity assessment. Resilience has been assessed as ‘Low’. Resistance as ‘High’. The sensitivity of this biotope to a change in emergence is considered as ‘Low’. | LowHelp | HighHelp | LowHelp |
Wave exposure changes (local) [Show more]Wave exposure changes (local)Benchmark. A change in near shore significant wave height of >3% but <5% for more than one year. Further detail EvidenceIR.MIR.KT.LdigT is predominantly recorded from wave sheltered sites, however is also recorded up to moderate wave exposure (Connor et al., 2004). The greatest wet weight of Laminaria digitata occurs at low wave exposure (mean significant wave height <0.4 m) decreasing by a mean of 83% in medium to high wave exposures (mean significant wave height >0.4 m; Gorman et al., 2013). At medium to high levels of wave exposure, Laminaria digitata biomass has been shown to decrease by 83% in the field (Wernberg and Thomsen, 2005). A flexible stipe and low profile holdfast allows Laminaria digitata to flourish in moderately to strongly wave exposed areas. In areas of high wave exposure Laminaria digitata may extend its upper limits into the lower eulittoral zone. However, IR.HIR.KFaR.Ala.Ldig typically replaces this biotope under conditions of extreme wave exposure, while in predominantly wave sheltered and lower water flow conditions IR.LIR.K.Slat.Ldig becomes prevalent. The physiology of seaweeds grown at exposed sites differs morphologically to those at sheltered sites with those exposed to greater wave action. A transplant experiment of Laminaria digitata, from exposed to sheltered sites resulted in a changed morphology with the frond widening, while individuals transplanted from sheltered to exposed sites became thinner more streamlined (Sundene, 1964; Gerard, 1987). This morphological plasticity is evident during the spore stage; because of this it is suggested that if wave height is increased or decreased the kelp with adapt morphologically over time to optimise its survival in the new environment. The associated assemblage of the biotope also influences Laminairia digitata’s ability to withstand increases in wave action. The epiphytic Membranipora membranacea reduces the ability of individual kelp to withstand wave action, increasing frond breakages and additionally reducing the maximum stress, toughness and extensibility of the kelp blade materials (Krumhansl et al., 2011). Sensitivity assessment. Wave exposure is one of the principal defining features of kelp biotopes, and large changes in wave exposure are likely to alter the relative abundance of the kelp species, grazing and understorey community, and hence, the biotope. However a change in near shore significant wave height of 3-5% is unlikely to have any significant effect on IR.MIR.KT.LdigT. Resistance has been assessed as ’High’, resilience as ‘High’ and sensitivity as ‘Not Sensitive’ at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Chemical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Transition elements & organo-metal contamination [Show more]Transition elements & organo-metal contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed but evidence is presented where available. No information was found concerning the specific effects of transitional elements on the characterizing or important functional flora and/or fauna of IR.MIR.KT.LdigT. The tolerance of Laminaria digitata to heavy metals is highly variable depending the on the metal concerned. Zinc was found to inhibit growth in Laminaria digitata at a concentration of 100 µg/L and at 515 µg/L, growth had almost completely ceased (Bryan, 1969). Axelsson & Axelsson (1987) investigated the effect of exposure to mercury (Hg), lead (Pb) and nickel (Ni) for 24 hours by measuring ion leakage to indicate plasma membrane damage. Inorganic and organic Hg concentrations of 1 mg/l resulted in the loss of ions equivalent to ion loss in seaweed that had been boiled for 5 minutes. Laminaria digitata was unaffected when subjected to Pb and Ni at concentrations up to 10 mg/l. The results also indicated that the species was intolerant of the tin compounds butyl-Sn and phenyl-Sn. Bryan (1984) suggested that the general order for heavy metal toxicity in seaweeds is: organic Hg > inorganic Hg > Cu > Ag > Zn > Cd > Pb. Cole et al. (1999) reported that Hg was very toxic to macrophytes and Boney (1971) reported that the red algae Plumaria elegans experienced 100% growth inhibition at 1 ppm Hg. However, no information was found concerning the specific effects of heavy metals on either Palmaria palmata or Corallina officinalis or any of the important faunal components of this biotope. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Hydrocarbon & PAH contamination [Show more]Hydrocarbon & PAH contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed but evidence is presented where available The brown algae are thought to be largely protected from oil penetration damage by the presence of a mucilaginous coating (O'Brian & Dixon, 1976). In addition, effects of oil accumulation on the thalli are mitigated by the perennial growth of kelps. Laminaria digitata is less susceptible to coating than some other seaweeds because of its preference for exposed locations where wave action will rapidly dissipate oil. The strong tidal flow in this biotope may provide some protection to all seaweeds within this community. No significant effects of the Amoco Cadiz spill were observed for Laminaria populations and the World Prodigy spill of 922 tons of oil in Narragansett Bay had no discernible effects on Laminaria digitata (Peckol et al., 1990). Furthermore, the upper limit of distribution for Laminaria digitata moved up wave exposed shores by as much as 2 m during the first few years after the Torrey Canyon oil spill due to the death of animals that graze the plants (Southward & Southward, 1978). Mesocosm studies in Norwegian waters showed that chronic low level oil pollution (25 µg/L) reduced growth rates in Laminaria digitata but only in the second and third years of growth (Bokn, 1985). O'Brien & Dixon (1976) suggested that red algae were the most sensitive group of algae to oil contamination. Where exposed to direct contact with fresh hydrocarbons, encrusting calcareous algae have a high intolerance. Crump et al. (1999) noted a dramatic bleaching on encrusting corallines and signs of bleaching in Corallina officinalis, Chondrus crispus and Mastocarpus stellatus at West Angle Bay, Pembrokeshire after the Sea Empress oil spill. However, encrusting corallines recovered quickly and Corallina officinalis was not killed. Laboratory studies of the effects of oil and dispersants on several red algae species, including Palmaria palmata (Grandy 1984, cited in Holt et al., 1995) concluded that they were all sensitive to oil/ dispersant mixtures, with little differences between adults, sporelings, diploid or haploid life stages. It is possible that Corallina officinalis and other algae are more intolerant of the dispersants used during oil spills than the oil itself. Where exposed to direct contact with fresh hydrocarbons, encrusting coralline algae appear to have a high intolerance. Crump et al. (1999) describe "dramatic and extensive bleaching" of 'Lithothamnia' following the Sea Empress oil spill. Observations following the Don Marika oil spill (K. Hiscock, own observations) were of rockpools with completely bleached coralline algae. However, Chamberlain (1996) observed that although Lithophyllum incrustans was quickly affected by oil during the Sea Empress spill, recovery occurred within about a year. The oil was found to have destroyed about one third of the thallus thickness but regeneration occurred from thallus filaments below the damaged area. No information was found concerning the specific effects of hydrocarbon contamination on the important faunal component of this biotope. However, the intolerance of the sponges, ascidians and bryozoans is likely to be related to depth of the oil / tar deposition and the strong tidal flow associated with this biotope is likely to reduce this. Nevertheless, analysis of kelp holdfast fauna after the Sea Empress oil spill in Milford Haven illustrated decreases in number of species, diversity and abundance at sites nearest the spill (SEEEC, 1998). | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Synthetic compound contamination [Show more]Synthetic compound contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed but evidence is presented where available. Laminaria digitata along with almost all red algal species and many animal species were found to be absent from sites close to acidified, halogenated effluent from a bromide extraction plant (Hoare & Hiscock, 1974). Axelsson & Axelsson (1987) investigated the effect on Laminaria digitata of exposure to various chemicals for 24 hours by measuring ion leakage as an indication of plasma membrane damage. However, only limited ion loss was seen on exposure to two detergents, nonylphenol ethoxylate (NP-10) and linear alkylbenzene sulfonate (LAS). Cole et al. (1999) suggested that herbicides such as Simazina and Atrazine were very toxic to macrophytic algae. Laboratory studies of the effects of oil and dispersants on several red algae species, including Palmaria palmata (Grandy, 1984, cited in Holt et al., 1995) concluded that they were all intolerant of oil/ dispersant mixtures, with little differences between adults, sporelings, diploid or haploid life stages. Smith (1968) reported that, in areas of heavy spraying of oil and detergent dispersants, Corallina officinalis was killed, and was affected down to a depth of 6 m in one site, presumably due to wave action and mixing. However, re-growth of fronds had begun within 2 months after spraying ceased. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Radionuclide contamination [Show more]Radionuclide contaminationBenchmark. An increase in 10µGy/h above background levels. Further detail EvidenceNo evidence | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction of other substances [Show more]Introduction of other substancesBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
De-oxygenation [Show more]De-oxygenationBenchmark. Exposure to dissolved oxygen concentration of less than or equal to 2 mg/l for one week (a change from WFD poor status to bad status). Further detail EvidenceReduced oxygen concentrations can inhibit both photosynthesis and respiration in macroalgae (Kinne, 1977). Despite this, macroalgae are thought to buffer the environmental conditions of low oxygen, thereby acting as a refuge for organisms in oxygen depleted regions especially if the oxygen depletion is short-term (Frieder et al., 2012). A rapid recovery from a state of low oxygen is expected if the environmental conditions are transient. If levels do drop below 4 mg/l negative effects on these organisms can be expected with adverse effects occurring below 2mg/l (Cole et al., 1999). The understorey faunal community may be affected by de-oxygenation, however IR.MIR.KT.LdigT is a tide swept biotope (Connor et al., 2004) and therefore the effects of de-oxygenation are likely to be short lived. Sensitivity Assessment. Reduced oxygen levels are likely to inhibit photosynthesis and respiration but not cause a loss of the macroalgae population directly. Resistance has been assessed as ‘High’, Resilience as ‘High’. Sensitivity has been assessed as ‘Not sensitive’ at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Nutrient enrichment [Show more]Nutrient enrichmentBenchmark. Compliance with WFD criteria for good status. Further detail EvidenceJohnston & Roberts (2009) conducted a meta analysis, which reviewed 216 papers to assess how a variety of contaminants (including sewage and nutrient loading) affected 6 marine habitats (including subtidal reefs). A 30-50% reduction in species diversity and richness was identified from all habitats exposed to the contaminant types. Johnston & Roberts (2009) however also highlighted that macro-algal communities are relative tolerant to contamination, but that contaminated communities can have low diversity assemblages which are dominated by opportunistic and fast growing species (Johnston & Roberts, 2009 and references therein). High ambient levels of phosphate and nitrogen enhance spore formation in a number of Laminaria spp. (Nimura et al., 2002), but will eventually inhibit spore production, particularly at the extremes of temperature tolerances as seen in Saccharina latissima (syn. Laminaria saccharina; Yarish et al., 1990). Laminaria digitata seems to follow this trend with a growth peak occurring in conjunction with nutrient generation from deeper waters in Norway (Gévaert et al., 2001). Despite this, enhancement of coastal nutrients is likely to favour those species with more rapid growth rates including turf forming algae (Gorgula & Connell, 2004). Sensitivity assessment. Although nutrients may not affect kelps directly, indirect effects such as turbidity may significantly affect photosynthesis. Furthermore organic enrichment may denude the associated community. However, the biotope is probably ‘Not sensitive’ (resistance is ‘High’ and resilience is ‘High) at the benchmark levels (i.e. compliance with WFD criteria). | HighHelp | HighHelp | Not sensitiveHelp |
Organic enrichment [Show more]Organic enrichmentBenchmark. A deposit of 100 gC/m2/yr. Further detail EvidenceJohnston & Roberts (2009) conducted a meta analysis, which reviewed 216 papers to assess how a variety of contaminants (including sewage and nutrient loading) affected 6 marine habitats (including subtidal reefs). A 30-50% reduction in species diversity and richness was identified from all habitats exposed to the contaminant types. Johnston & Roberts (2009) however also highlighted that macro-algal communities are relative tolerant to contamination, but that contaminated communities can have low diversity assemblages which are dominated by opportunistic and fast growing species (Johnston & Roberts, 2009 and references therein). Macroalgal growth is generally nitrogen-limited in the summer, as illustrated by increased growth rates of Laminaira digitata in a eutrophic site when compared to an oligotrophic in Abroath, Scotland (Davison et al., 1984). The deposition of sewage effluent into coastal environments resulted in the absence of Laminaria digitata from the coastline of the Firth of Forth (Read et al., 1983) although this was probably coupled with the decrease in water clarity also observed at the time. The use of some kelp species in conjunction with fish aquaculture (buffering the effects of organic enrichment in the local area) suggests that many commercial kelps (including Laminaria digitata) are tolerant to increased levels of ammonia and faecal matter, dependent on fish species and aquaculture design (Troell et al., 2003). Sensitivity assessment. Although organic enrichment may not affect kelps directly, indirect effects such as turbidity may significantly affect photosynthesis. Furthermore organic enrichment may denude the associated community. Resistance has therefore been assessed as ‘Medium’, resilience as ‘High’. Sensitivity has been assessed as ’Low’. | MediumHelp | HighHelp | LowHelp |
Physical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Physical loss (to land or freshwater habitat) [Show more]Physical loss (to land or freshwater habitat)Benchmark. A permanent loss of existing saline habitat within the site. Further detail EvidenceAll marine habitats and benthic species are considered to have a resistance of ‘None’ to this pressure and to be unable to recover from a permanent loss of habitat (resilience is ‘Very Low’). Sensitivity within the direct spatial footprint of this pressure is therefore ‘High’. Although no specific evidence is described confidence in this assessment is ‘High’, due to the incontrovertible nature of this pressure. | NoneHelp | Very LowHelp | HighHelp |
Physical change (to another seabed type) [Show more]Physical change (to another seabed type)Benchmark. Permanent change from sedimentary or soft rock substrata to hard rock or artificial substrata or vice-versa. Further detail EvidenceIf rock substrata were replaced with sedimentary substrata this would represent a fundamental change in habitat type, which kelp species would not be able to tolerate (Birkett et al., 1998). The biotope would be lost. Sensitivity assessment. Resistance to the pressure is considered ‘None’, and resilience ‘Very low’ or ‘None’. The sensitivity of this biotope to change from sedimentary or soft rock substrata to hard rock or artificial substrata or vice-versa is assessed as ‘High’. | NoneHelp | Very LowHelp | HighHelp |
Physical change (to another sediment type) [Show more]Physical change (to another sediment type)Benchmark. Permanent change in one Folk class (based on UK SeaMap simplified classification). Further detail EvidenceNot relevant | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Habitat structure changes - removal of substratum (extraction) [Show more]Habitat structure changes - removal of substratum (extraction)Benchmark. The extraction of substratum to 30 cm (where substratum includes sediments and soft rock but excludes hard bedrock). Further detail EvidenceNot relevant | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Abrasion / disturbance of the surface of the substratum or seabed [Show more]Abrasion / disturbance of the surface of the substratum or seabedBenchmark. Damage to surface features (e.g. species and physical structures within the habitat). Further detail EvidenceAbrasion of the substratum e.g. from bottom or pot fishing gear, cable laying etc. may cause localized mortality of the resident community. The effect would be situation dependant however if bottom fishing gear were towed over a site it may cause high mortality in the resident community and potentially remove areas of the kelp. Sensitivity assessment. Resistance has been assessed as ‘None’, resilience as ‘High’. Sensitivity has been assessed as ‘Medium’. | NoneHelp | HighHelp | MediumHelp |
Penetration or disturbance of the substratum subsurface [Show more]Penetration or disturbance of the substratum subsurfaceBenchmark. Damage to sub-surface features (e.g. species and physical structures within the habitat). Further detail EvidenceNot relevant | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Changes in suspended solids (water clarity) [Show more]Changes in suspended solids (water clarity)Benchmark. A change in one rank on the WFD (Water Framework Directive) scale e.g. from clear to intermediate for one year. Further detail EvidenceSuspended Particle Matter (SPM) concentration has a linear relationship with sub surface light attenuation (Kd) (Devlin et al., 2008). Light availability and water turbidity are principal factors in determining depth range at which kelp can be found (Birkett et al., 1998). Light penetration influences the maximum depth at which kelp species can grow and it has been reported that laminarians grow down to depths at which the light levels are reduced to one percent of incident light at the surface. Maximal depth distribution of laminarians therefore varies from 100 m in the Mediterranean to only 6-7 m in the silt laden German Bight. In Atlantic European waters, the depth limit is typically 35 m. In very turbid waters the depth at which kelp is found may be reduced, or in some cases excluded completely (e.g. Severn Estuary), because of the alteration in light attenuation by suspended sediment (Lüning, 1990; Birkett et al. 1998). Laminaria spp. show a decrease of 50% photosynthetic activity when turbidity increases by 0.1/m (light attenuation coefficient = 0.1-0.2/m; Staehr & Wernberg, 2009). An increase in water turbidity will likely affect the photosynthetic ability of Laminaria digitata, decrease kelp abundance and density. An increase in SPM results in a decrease in sub-surface light attenuation. The absence of Laminaria digitata in the Firth of Forth was suggested to be caused by the outflow from a sewage treatment plant, which increased the turbidity of the water and thus decreased photosynthetic activity, although the effect of turbidity was probably coupled with increased nutrient levels (Read et al., 1983). In locations where water clarity is severely decreased, Laminaria species experience a significant decrease in growth from the shading of suspended matter and/or phytoplankton (Lyngby & Mortensen 1996, Spilmont et al., 2009). Sensitivity Assessment. A decrease in turbidity is likely to support enhanced growth (and possible habitat expansion) and is therefore not considered in this assessment. However, an increase in water clarity from clear to intermediate (10-100 mg/l) represents a change in light attenuation of ca 0.67-6.7 Kd/m, and is likely to result in a greater than 50% reduction in photosynthesis of Laminaria spp. Therefore, the dominant kelp species will probably suffer a significant decline and resistance to this pressure is assessed as ‘Low’. Resilience to this pressure is probably High at the benchmark. Hence, this biotope is assessed as having a sensitivity of Low to this pressure. | LowHelp | HighHelp | LowHelp |
Smothering and siltation rate changes (light) [Show more]Smothering and siltation rate changes (light)Benchmark. ‘Light’ deposition of up to 5 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceLaminaria digitata is more sensitive to this pressure than other subtidal brown algae (e.g. Sargassum muticum; Morrell & Furnhan, 1982). A layer of fine grained sediment (0.1-0.2cm thick) caused rotting of the plant and 25% mortality after 4 weeks of coverage in a laboratory experiment suggesting that in locations of low wave and current mediated water flow, sedimentation is a threat to this biotope (Lyngby & Mortensen, 1996). The abundance of filter feeders may experience some short lived interference with their feeding apparatus and respiratory flows. Furthermore, the holdfasts and lower end of the stipes of Laminaria digitata may experience some mild sand scour. IR.MIR.KT.LdigT is however a tide swept biotope, recorded from very strong to very weak tidal streams (negligible->3m/s). High water movement is likely to remove sediment within a couple of tidal cycles. Sensitivity assessment. Resistance has been assessed as ‘High’, resilience as ‘High’. Sensitivity has been assessed as ‘Not sensitive’. | HighHelp | HighHelp | Not sensitiveHelp |
Smothering and siltation rate changes (heavy) [Show more]Smothering and siltation rate changes (heavy)Benchmark. ‘Heavy’ deposition of up to 30 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceLaminaria digitata is more sensitive to this pressure than other subtidal brown algae (e.g. Sargassum muticum; Morrell & Furnhan, 1982). A layer of fine grained sediment (0.1-0.2cm thick) caused rotting of the plant and 25% mortality after 4 weeks of coverage in a laboratory experiment suggesting that in locations of low wave and current mediated water flow, sedimentation is a threat to this biotope (Lyngby & Mortensen, 1996). The abundance of filter feeders may experience some short lived interference with their feeding apparatus and respiratory flows. Furthermore, the holdfasts and lower end of the stipes of Laminaria digitata may experience some mild sand scour. IR.MIR.KT.LdigT is however a tide swept biotope, recorded from very strong to very weak tidal streams (negligible->3m/s). High water movement is likely to remove sediment within a few of tidal cycles. Sensitivity assessment. Due to the volume of deposited sediment within this pressure it is anticipated the sediment will be retained within the host habitat longer than with “light” deposition. Sediment retention may therefore inundate filter feeding fauna and cause Laminaria digitata mortality. Resistance has been assessed as ‘Low’, resilience as ‘High’. Sensitivity has been assessed as ‘Low’. | LowHelp | HighHelp | LowHelp |
Litter [Show more]LitterBenchmark. The introduction of man-made objects able to cause physical harm (surface, water column, seafloor or strandline). Further detail EvidenceNot assessed. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Electromagnetic changes [Show more]Electromagnetic changesBenchmark. A local electric field of 1 V/m or a local magnetic field of 10 µT. Further detail EvidenceNo evidence. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Underwater noise changes [Show more]Underwater noise changesBenchmark. MSFD indicator levels (SEL or peak SPL) exceeded for 20% of days in a calendar year. Further detail EvidenceNot relevant. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Introduction of light or shading [Show more]Introduction of light or shadingBenchmark. A change in incident light via anthropogenic means. Further detail EvidenceThere is no evidence to suggest that anthropogenic light sources would affect Laminaria digitata. Shading of the biotope (e.g. by construction of a pontoon, pier etc) could adversely affect the biotope in areas where the water clarity is also low, and tip the balance to shade tolerant species, resulting in the loss of the biotope directly within the shaded area, or a reduction in laminarian abundance from forest to park type biotopes. Sensitivity assessment. Resistance is 'Low', with a 'High' resilience and a sensitivity of 'Low', albeit with 'low' confidence due to the lack of direct evidence. | LowHelp | HighHelp | LowHelp |
Barrier to species movement [Show more]Barrier to species movementBenchmark. A permanent or temporary barrier to species movement over ≥50% of water body width or a 10% change in tidal excursion. Further detail EvidenceNot relevant. This pressure is considered applicable to mobile species, e.g. fish and marine mammals rather than seabed habitats. Physical and hydrographic barriers may limit the dispersal of spores. But spore dispersal is not considered under the pressure definition and benchmark. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Death or injury by collision [Show more]Death or injury by collisionBenchmark. Injury or mortality from collisions of biota with both static or moving structures due to 0.1% of tidal volume on an average tide, passing through an artificial structure. Further detail EvidenceNot relevant | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Visual disturbance [Show more]Visual disturbanceBenchmark. The daily duration of transient visual cues exceeds 10% of the period of site occupancy by the feature. Further detail EvidenceNot relevant | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Biological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Genetic modification & translocation of indigenous species [Show more]Genetic modification & translocation of indigenous speciesBenchmark. Translocation of indigenous species or the introduction of genetically modified or genetically different populations of indigenous species that may result in changes in the genetic structure of local populations, hybridization, or change in community structure. Further detail EvidenceNo evidence regarding the genetic modification of Laminaria digitata was found. Although Laminaria digitata is harvested, cultivation appears to be done on wild kelp stands in a sustainable 5-year cycle (Vea & Ask, 2011), therefore, translocation of Laminaria digitata is unlikely. In addition, if the translocation of populations did occur, a loss in genetic diversity is not regarded as an issue for Laminaria digitata, unless additional pressures resulted in the isolation and fragmentation of wild coastal populations (Valero et al., 2011). | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction of microbial pathogens [Show more]Introduction of microbial pathogensBenchmark. The introduction of relevant microbial pathogens or metazoan disease vectors to an area where they are currently not present (e.g. Martelia refringens and Bonamia, Avian influenza virus, viral Haemorrhagic Septicaemia virus). Further detail EvidenceLaminaria digitata may be infected by the microscopic brown alga Streblonema aecidioides. Infected algae show symptoms of Streblonema disease, i.e. alterations of the blade and stipe ranging from dark spots to heavy deformations and completely crippled thalli (Peters & Scaffelke, 1996). Infection can reduce growth rates of host algae. Sensitivity assessment. Resistance to the pressure is considered ‘Medium’, and resilience ‘High’. The sensitivity of this biotope to introduction of microbial pathogens is assessed as ‘Low’. | MediumHelp | HighHelp | LowHelp |
Removal of target species [Show more]Removal of target speciesBenchmark. Removal of species targeted by fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidenceTraditionally Laminaria digitata was used on agricultural lands as fertilizers; now Laminaria spp. are used in a range of different products, with its alginates used in the cosmetic, pharmaceutical and agri-food industries (Kervarec et al., 1999; McHugh, 2003). Laminaria digitata is harvested with a ‘Scoubidou’ (a curved iron hook which is mechanically operated). This device is considered to be selective- only harvesting individuals older than 2 years (Arzel, 2002). France reportedly harvests 75,000t kelp, mainly consisting of Laminaria digitata annually (FAO, 2007). Canopy removal of Laminaria digitata has been shown to reduce shading, resulting in the bleaching of sub canopy algae (Hawkins & Harkins, 1985). Harvesting may also result in habitat fragmentation, a major threat to this biotope’s ecosystem functioning (Valero et al., 2011). Maintaining a sustainable crop of Laminaria digitata has been suggested as possible if the industry continues employing small vessels evenly dispersed along the coastline. This would protect against habitat fragmentation and buffer over exploitation (Davoult et al., 2011). A fallow period of 18-24 months has been suggested for Laminaria digitata in France, where competition between the juvenile sporophytes of Laminaria digitata and Saccorhiza polyschides has been indicated as a threat to the continued harvesting effort of Laminaria digitata (Engelen et al., 2011). Sensitivity Assessment. Resistance has been assessed as ‘Low’, resilience ‘High’. Sensitivity has been assessed as ‘Low’. | LowHelp | HighHelp | LowHelp |
Removal of non-target species [Show more]Removal of non-target speciesBenchmark. Removal of features or incidental non-targeted catch (by-catch) through targeted fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidenceIncidental/accidental removal of Laminaria digitata as a result of non-targeted fisheries practices is likely to have a significant effect on the ecology of IR.MIR.KT.LdigT. Targeted Laminaria hyperborea trawling in southern Norway is reported to remove all canopy forming sporophytes (Christie et al. 1998). Sensitivity assessment. Resistance has been assessed as ‘None’, resilience as ‘High’ and sensitivity as ‘Medium’. | NoneHelp | HighHelp | MediumHelp |
Introduction or spread of invasive non-indigenous species (INIS) Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Other INIS [Show more]Other INISEvidenceCompetition with invasive macroalgae may be a potential threat to this biotope. Potential invasives include Undaria pinnatifida, Sargassum muticum and Codium fragile spp. tormentosoides. In Nova Scotia, Codium fragile spp. tormentosoides competes successfully with native kelps for space including Laminaria digitata, exploiting gaps within the kelp beds. Once established the algal mat created by Codium fragile spp. tormentosoides prevents re-colonization by other macro-algae (Scheibling & Gagnon, 2006). Sargassum muticum is a circumglobal invasive species (Engelen et al., 2015). It is recorded (2015) from Norway to Morocco and into the Mediterranean in the eastern Atlantic, and from Alaska to Baja California in the eastern Pacific, and from southern Russia to southern China in the western Pacific (Engelen et al., 2015). It colonizes a variety of habitats and can tolerate -1°C to 30°C and survive salinities below 10 ppt. Although fertilization does not occur below 15 ppt and growth of germlings is limited below 10°C, it can complete its life cycle as long as temperatures are over 8°C for at least four months of the year (Engelen et al., 2015). However, its distribution is limited by the availability of hard substratum (e.g. stones >10 cm) and light (Staeher et al., 2000; Strong & Dring 2011; Engelen et al., 2015). It is most abundant between 1 and 3 m below mean water. But it has been recorded at 18 m or 30 m in the clear waters of California. However, it is a poor competitor under low light and only develops dense canopies in shallow areas (Engelen et al., 2015). Sargassum muticum was shown to replace and out-compete leathery, canopy-forming macroalgae such as Saccharina latissima, Halidrys siliquosa, and Fucus spp. and, to a lesser degree, understorey species such as Codium fragile, Chondrus crispus and Dictyota dichotoma in Limfjorden, Denmark between 1984 and 1997 (Staehr et al., 2000; Engelen et al., 2015; de Bettignies et al., 2021). The invasion in Limfjorden had stabilized by 2005 although many of the native macroalgal species continued to decline (Engelen et al., 2015). In Limfjorden, the distribution of Sargassum muticum was limited to areas with hard substratum, in particular stones >10 cm in diameter, while smaller stones, gravel and sand were unsuitable. It was most abundant between 1 and 4 m in depth but had low cover at 0 to 0.5 m or 4 to 6 m, in the turbid waters of the Limfjorden. Limfjorden is wave sheltered although wave exposure has been reported to restrict the growth and survival of Sargassum muticum (Staehr et al., 2000). Viejo et al. (1995) reported that Sargassum muticum transplanted to wave exposed shores in Spain experienced >80% breakages within a month and that the growth of undamaged plants was significantly lower than that of plants on sheltered shores. Similarly, Andrew & Viejo (1998) noted that Sargassum muticum was restricted to intertidal rockpools in wave exposed sites in the Bay of Biscay. Strong & Dring (2011) used canopy removal experiments to investigate inter- and intra-species competition between Sargassum muticum and Saccharina latissima in the Dorn, Strangford Lough, N. Ireland. The Dorn consists of tidal pools, very sheltered from wave action but with moderately strong tidal streams (1-2 knots). Sargassum muticum grew better in mixed stands with Saccharina latissima than in the highest density monospecific stands examined. However, the growth of Saccharina was not affected by the proportion of Sargassum in mixed stands. They concluded that Saccharina was not impacted significantly by the alien species while Sargassum benefited from growth in mixed stands. Experimental manipulation of subtidal algal canopies in San Juan Islands, Washington State, USA, showed that Sargassum muticum reduced the abundance of native macroalgae, including the kelp Laminaria bongardiana due to shading. However, the experimental removal of Sargassum resulted in the recovery of native species within about 1 year (Britton-Simmons, 2004; Engelen et al., 2015). The negative effects of Sargassum muticum on native macroalgae are mainly due to competition for light, rather than changes in nutrient availability, sedimentation or water flow (Britton-Simmons, 2004; Engelen et al., 2015). Cosson (1999) reported a significant decline in Laminaria digitata at two sites between 1983 and 1997 on the coast of Normandy, France, due to an increase in Sargassum muticum abundance in the same areas. For example, on the Grandcamp rocks, Laminaria digitata has almost disappeared while Sargassum muticum had covered 80% of the lower intertidal and subtidal zone in summer. Undaria pinnatifida (Wakame or Asian kelp) is a large brown seaweed and an Invasive Non-Indigenous Species (INIS) that could out-compete native UK kelp species (see Farrell & Fletcher, 2006; Thompson & Schiel, 2012; Brodie et al., 2014; Hieser et al., 2014; Arnold et al., 2016; Epstein & Smale, 2017; Epstein & Smale, 2018; Kraan, 2017; Epstein et al., 2019a,b; Tidbury, 2020). Undaria pinnatifida originates from Japan but is established currently on the coastlines of New Zealand, Australia, Northern France, Spain, Italy, the UK, Portugal, Belgium, Holland, Argentina, Mexico, and the USA (De Leij et al., 2017). Undaria pinnatifida was first recorded in the UK in the Hamble Estuary in 1994 (Macleod et al., 2016). It has since proliferated along UK coastlines. One year after its discovery at the Queen Anne Battery marina, Plymouth, it became a major fouling plant on pontoons (Minchin & Nunn, 2014). Although initially restricted to artificial habitats, such as marinas and ports, it is now widespread in natural habitats in several areas, including Plymouth Sound. Undaria pinnatifida seems to settle better on artificial substrata (e.g. floats, marinas or piers) than on natural rocky shores among local kelps (Vaz-Pinto et al., 2014). It is found predominantly in low intertidal to shallow subtidal habitats (Epstein et al., 2019b) and is significantly more abundant on artificial substrata compared to natural rocky substrata (Heiser et al., 2014; Epstein & Smale, 2018). James (2017) suggested that Undaria pinnatifida could out-compete native species on artificial substrata (such as marinas and wharf structures). In Plymouth, UK, De Leij et al. (2017) found that natural habitats with dense native macroalgal canopies, such as Laminaria hyperborea, Laminaria ochroleuca, Laminaria digitata and Saccharina latissima had more resistance to Undaria pinnatifida invasion than disturbed or sparse canopies, due to limited space and light availability for Undaria pinnatifida recruits. However, the dense canopies did not always prevent invasion of Undaria pinnatifida as sporophytes were still recorded within dense Laminaria canopies, so that canopy disturbance was not always required (De Leij et al., 2017; Epstein & Smale, 2018. Undaria pinnatifida species behaves as a winter annual and recruitment occurs in winter followed by rapid growth through spring, maturity, and then senescence through summer, with only the microscopic life stages persisting through autumn. It exhibits multiple dispersal strategies, such as short-range spore dispersal, and long-range dispersal as whole drift plants or fragments. Undaria pinnatifida has spread rapidly across the UK and Europe, resulting in community-wide responses and impacts (Vaz-Pinto et al., 2014; Epstein & Smale, 2017). Its impacts are complex and context-specific, depending on space, time, and taxa present in the introduced location (Epstein & Smale, 2017; Teagle et al., 2017; Tidbury, 2020). Undaria pinnatifida has a wide physiological niche meaning it can occur in both coastal and estuarine environments showing tolerance for varying salinities, turbidity and siltation (Heiser et al., 2014; Epstein & Smale, 2018). Undaria pinnatifida has a preference for sites sheltered with low wave exposure and weak tidal streams (Heiser et al., 2014; Epstein & Smale, 2018). In natural habitats, Undaria pinnatifida was not recorded if the wave fetch was greater than 642 km but increased in abundance and cover in very sheltered sites (Epstein & Smale, 2018). In St Malo, France, there was evidence that Undaria pinnatifida could co-exist with Laminaria digitata under certain conditions (Castric-Fey et al., 1993). Epstein et al. (2019b) observed that, in Plymouth Sound, UK, Undaria pinnatifida co-existed with seven species of canopy-forming brown macroalgae within its depth range (+1 to –4 m), including Laminaria digitata; which suggested that they could occupy an overlapping niche. Epstein & Smale (2018) also observed that Undaria pinnatifida was relatively common (abundance of >70 individuals per 25 m transect) at three sites in Devon, UK (Jennycliff, Bovisand and Beacon Cove) where Laminaria spp. were abundant (40-79%) or superabundant (>80%), which suggested that Undaria pinnatifida could co-exist within refugia amongst areas with dense Laminaria spp.. In many cases, Undaria pinnatifida seems to have minimal impacts on native communities (e.g. Forrest & Taylor, 2002; Valentine & Johnson, 2003; South et al., 2016; Epstein & Smale, 2017; Epstein & Smale 2018). Laminaria digitata forms dense monospecific canopies and its thick, extensive laminae are likely to restrict light penetration to the underlying substratum, including Undaria pinnatifida (De Leij et al., 2017). Disturbance to the native kelp canopy can facilitate the spread of INIS by increasing the availability of resources such as light and space. Experimental full removal of the existing kelp canopy in Plymouth Sound allowed the mean cover of Undaria pinnatifida to increase from ca 10% to ca 50% within three months (De Leij et al.,2017). Their experiment showed that the density of Laminaria digitata was important to Undaria pinnatifida invasion (De Leij et al., 2017). Similarly, a primary succession experiment by Epstein et al. (2019b) in Plymouth Sound (UK) showed that clearance of Laminaria digitata in 2016 resulted in an increase in Undaria pinnatifida abundance. However, this was quickly followed by the recovery of Laminaria digitata in 2017 and the concurrent decline in Undaria pinnatifida, which suggested that Laminaria digitata had a higher fitness (Epstein et al., 2019b). Within the same study, Epstein et al. (2019b) observed that Undaria pinnatifida exhibited a significant negative relationship with Laminaria digitata on intertidal rocky reef substrata, which suggested that Laminaria digitata negatively affected Undaria pinnatifida abundance. It was also suggested that Undaria pinnatifida has a lower resistance to desiccation than Laminaria digitata. As a result, Epstein & Smale (2019b) concluded that due to its lower fitness, Undaria found within natural habitats in the northeast Atlantic has low ecological and community level impacts and was competitively inferior to Laminaria spp. However, Heiser et al. (2014) found that in Plymouth, UK, Laminaria digitata was significantly less abundant at sites with the presence of Undaria pinnatifida, with only ca 1.5 Laminaria digitata individuals per m2 with Undaria pinnatifida, compared to ca 7 individuals per m2 at sites without Undaria pinnatifida. Undaria pinnatifida was successfully eradicated on a sunken ship in Clatham Islands, New Zealand, by applying a heat treatment of 70°C (Wotton et al., 2004). However, numerous other eradication attempts have failed and, as noted by Fletcher & Farrell (1998), once established Undaria pinnatifida resists most attempts at long-term removal. Sensitivity Assessment. The above evidence suggests that Undaria pinnatifida can co-exist with native kelp species within its depth range (-1 to 4 m) e.g. Laminaria digitata as shown in Plymouth Sound, UK. The dense kelp canopy may restrict or slow the proliferation of Undaria pinnatifida. But, Laminaria digitata has been shown to out-compete Undaria pinnatifida. For example, removal of the Laminaria digitata canopy resulted in the increase of Undaria pinnatifida abundance but recovery of Laminaria digitata in the following year resulted in a concurrent decline in Undaria pinnatifida. This Laminaria digitata dominated biotope (IR.MIR.KT.LdigT) is found in the sublittoral fringe sheltered from wave action but structured by strong tidal streams. The evidence above suggests that Undaria prefers sheltered conditions, with low tidal flow, and is less tolerant of desiccation than Laminaria digitata. It is unlikely to out-compete or replace Laminaria digitata under the physical conditions that characterize this biotope. However, Sargassum muticum prefers wave sheltered, shallow sites in the sublittoral fringe, and has been reported to invade and out-compete Laminaria digitata beds in France (Cosson, 1999; de Bettignies et al., 2021). Therefore, resistance is assessed as ‘Low’ to represent the possibility of colonization by Sargassum. While Laminaria digitata may out-compete Undaria the recovery after invasion by Sargassum, although rapid, would require direct intervention (removal). Hence, resilience is probably ‘Very low’ so sensitivity is assessed as ‘High’. Overall, confidence is assessed as ‘Low’ due to evidence of variation and site-specific nature of competition between native kelps and both Undaria pinnatifida and Sargassum muticum and the limited direct evidence of the effect of Sargassum on Laminaria digitata. | LowHelp | Very LowHelp | HighHelp |
Bibliography
Abelson, A., Weihs, D. & Loya, Y., 1994. Hydrodynamic impediments to settlement of marine propagules, and adhesive-filament solutions. Limnology and Oceanography, 39, 164-169.
Andrew, N.L. & Viejo, R.M., 1998. Ecological limits to the invasion of Sargassum muticum in northern Spain. Aquatic Botany, 60 (3), 251-263. DOI https://doi.org/10.1016/S0304-3770(97)00088-0
Arafeh-Dalmau, N., Montaño-Moctezuma, G., Martínez, J.A., Beas-Luna, R., Schoeman, D.S. & Torres-Moye, G., 2019. Extreme Marine Heatwaves Alter Kelp Forest Community Near Its Equatorward Distribution Limit. Frontiers in Marine Science, 6 (499). DOI https://doi.org/10.3389/fmars.2019.00499
Arnold, M., Teagle, H., Brown, M.P. & Smale, D.A., 2016. The structure of biogenic habitat and epibiotic assemblages associated with the global invasive kelp Undaria pinnatifida in comparison to native macroalgae. Biological Invasions, 18 (3), 661-676. DOI https://doi.org/10.1007/s10530-015-1037-6
Arzel, P., 1998. Les laminaires sur les côtes bretonnes. Évolution de l'exploitation et de la flottille de pêche, état actuel et perspectives. Plouzané, France: Ifremer.
Assis, J., Lucas, A.V., Bárbara, I. & Serrão, E.Á., 2016. Future climate change is predicted to shift long-term persistence zones in the cold-temperate kelp Laminaria hyperborea. Marine Environmental Research, 113, 174-182. DOI https://doi.org/10.1016/j.marenvres.2015.11.005
Assis, J., Serrão, E.A., Claro, B., Perrin, C. & Pearson, G.A., 2014. Climate-driven range shifts explain the distribution of extant gene pools and predict future loss of unique lineages in a marine brown alga. Molecular Ecology, 23 (11), 2797-2810. DOI https://doi.org/10.1111/mec.12772
Axelsson, B. & Axelsson, L., 1987. A rapid and reliable method to quantify environmental effects on Laminaria based on measurements of ion leakage. Botanica Marina, 30, 55-61.
Barthel, D. & Wolfrath, B., 1989. Tissue sloughing in the sponge Halichondria panicea: a fouling organism prevents being fouled. Oecologia, 78, 357-360.
Barthel, D., 1988. On the ecophysiology of the sponge Halichondria panicea in Kiel Bight. II. Biomass, production, energy budget and integration in environmental processes. Marine Ecology Progress Series, 43, 87-93.
Beaver, R. & Dipper, F.A., 2002. Marine Nature Conservation Review Sector 14. Sealochs in the Outer Hebrides: Area summaries. Peterborough: Joint Nature Conservation Committee.
Birkett, D.A., Maggs, C.A., Dring, M.J. & Boaden, P.J.S., 1998b. Infralittoral reef biotopes with kelp species: an overview of dynamic and sensitivity characteristics for conservation management of marine SACs. Natura 2000 report prepared by Scottish Association of Marine Science (SAMS) for the UK Marine SACs Project., Scottish Association for Marine Science. (UK Marine SACs Project, vol VI.), 174 pp. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/reefkelp.pdf
Blight, A.J. & Thompson, R.C., 2008. Epibiont species richness varies between holdfasts of a northern and a southerly distributed kelp species. Journal of the Marine Biological Association of the United Kingdom, 88 (03), 469-475.
Bokn, T., 1985. Effects of diesel oil on commercial benthic algae in Norway. In Proceedings of 1985 Oil Spill Conference, (ed. American Petroleum Institute), pp. 491-496. Washington, D.C.: American Petroleum Institute.
Bolton, J.J. & Lüning, K.A.F., 1982. Optimal growth and maximal survival temperatures of Atlantic Laminaria species (Phaeophyta) in culture. Marine Biology, 66, 89-94.
Boney, A.D., 1971. Sub-lethal effects of mercury on marine algae. Marine Pollution Bulletin, 2, 69-71.
Brazier, D.P., Holt, R.H.F., Murray, E. & Nichols, D.M., 1999. Marine Nature Conservation Review Sector 10. Cardigan Bay and North Wales: area summaries. Peterborough: Joint Nature Conservation Committee. [Coasts and seas of the United Kingdom. MNCR Series.]
Breeman, A.M., 1990. Expected Effects of Changing Seawater Temperatures on the Geographic Distribution of Seaweed Species. In Beukema, J.J., et al. (eds.). Expected Effects of Climatic Change on Marine Coastal Ecosystems, Dordrecht: Springer Netherlands, pp. 69-76. DOI: https://doi.org/10.1007/978-94-009-2003-3_9
Britton, D., Cornwall, C.E., Revill, A.T., Hurd, C.L. & Johnson, C.R., 2016. Ocean acidification reverses the positive effects of seawater pH fluctuations on growth and photosynthesis of the habitat-forming kelp, Ecklonia radiata. Scientific reports, 6 (1), 26036. DOI: https://doi.org/10.1038/srep26036
Britton-Simmons, K.H., 2004. Direct and indirect effects of the introduced alga Sargassum muticum on benthic, subtidal communities of Washington State, USA. Marine Ecology Progress Series, 277, 61-78. DOI https://doi.org/10.3354/meps277061
Brodie J., Williamson, C.J., Smale, D.A., Kamenos, N.A., Mieszkowska, N., Santos, R., Cunliffe, M., Steinke, M., Yesson, C. & Anderson, K.M., 2014. The future of the northeast Atlantic benthic flora in a high CO2 world. Ecology and Evolution, 4 (13), 2787-2798. DOI https://doi.org/10.1002/ece3.1105
Bryan, G.W., 1969. The absorption of zinc and other metals by the brown seaweed Laminaria digitata. Journal of the Marine Biological Association of the United Kingdom, 49, 225-243.
Bryan, G.W., 1984. Pollution due to heavy metals and their compounds. In Marine Ecology: A Comprehensive, Integrated Treatise on Life in the Oceans and Coastal Waters, vol. 5. Ocean Management, part 3, (ed. O. Kinne), pp.1289-1431. New York: John Wiley & Sons.
Castric-Fey, A., Girard, A. & L'Hardy-Halos, M.T., 1993. The Distribution of Undaria pinnatifida (Phaeophyceae, Laminariales) on the Coast of St. Malo (Brittany, France). Botanica Marina, 36 (4), 351-358. DOI https://doi.org/10.1515/botm.1993.36.4.351
Cazenave, A. & Nerem, R.S., 2004. Present-day sea-level change: Observations and causes. Reviews of Geophysics, 42 (3). DOI https://doi.org/10.1029/2003rg000139
Chamberlain, Y.M., 1996. Lithophylloid Corallinaceae (Rhodophycota) of the genera Lithophyllum and Titausderma from southern Africa. Phycologia, 35, 204-221.
Chapman, A.R.O., 1981. Stability of sea urchin dominated barren grounds following destructive grazing of kelp in St. Margaret's Bay, Eastern Canada. Marine Biology, 62, 307-311.
Christie, H., Fredriksen, S. & Rinde, E., 1998. Regrowth of kelp and colonization of epiphyte and fauna community after kelp trawling at the coast of Norway. Hydrobiologia, 375/376, 49-58.
Church, J.A. & White, N.J., 2006. A 20th century acceleration in global sea-level rise. Geophysical Research Letters, 33 (1). DOI https://doi.org/10.1029/2005gl024826
Church, J.A., White, N.J., Coleman, R., Lambeck, K. & Mitrovica, J.X., 2004. Estimates of the Regional Distribution of Sea Level Rise over the 1950–2000 Period. Journal of Climate, 17 (13), 2609-2625.
Cole, S., Codling, I.D., Parr, W. & Zabel, T., 1999. Guidelines for managing water quality impacts within UK European Marine sites. Natura 2000 report prepared for the UK Marine SACs Project. 441 pp., Swindon: Water Research Council on behalf of EN, SNH, CCW, JNCC, SAMS and EHS. [UK Marine SACs Project.]. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/water_quality.pdf
Cosson, J., 1999. Sur la disparition progressive de Laminaria digitata sur les cotes du Calvados (France). Cryptogamie: Algol, 20, 35-42.
Crisp, D.J. (ed.), 1964. The effects of the severe winter of 1962-63 on marine life in Britain. Journal of Animal Ecology, 33, 165-210.
Crump, R.G., Morley, H.S., & Williams, A.D., 1999. West Angle Bay, a case study. Littoral monitoring of permanent quadrats before and after the Sea Empress oil spill. Field Studies, 9, 497-511.
Davies, C.E. & Moss, D., 1998. European Union Nature Information System (EUNIS) Habitat Classification. Report to European Topic Centre on Nature Conservation from the Institute of Terrestrial Ecology, Monks Wood, Cambridgeshire. [Final draft with further revisions to marine habitats.], Brussels: European Environment Agency.
Davison, I.R., Andrews, M. & Stewart, W.D.P., 1984. Regulation of growth in Laminaria digitata: use of in-vivo nitrate reductase activities as an indicator of nitrogen limitation in field populations of Laminaria spp. Marine Biology, 84, 207-217.
De Bettignies, T., de Bettignies, F., Bartsch, I., Bekkby, T., Boiffin, A., Casado de Amezúa, P., Christie, H., Edwards, H., Fournier, N., García, A., Gauthier, L., Gillham, K., Halling, C., Harrald, M., Hennicke, J., Hernández, S., Kilnäs, M., Martinez, B., Mieszkowska, N., Moore, P., Moy, F., Mueller, M., Norderhaug, K.M., Ó Cadhla, O., Parry, M., Ramsay, K., Robertson, M., Russel, T., Serrão, E., Smale, D., Sousa Pinto, I., Steen, H., Street, M., Walday, M., Werner, T. & La Rivière, M., 2021. Background Document for Kelp Forests. OSPAR Commission, London, OSPAR 788/2021, 66 pp. Available from: https://www.ospar.org/documents?v=46796
De Leij, R., Epstein, G., Brown, M.P. & Smale, D.A., 2017. The influence of native macroalgal canopies on the distribution and abundance of the non-native kelp Undaria pinnatifida in natural reef habitats. Marine Biology, 164 (7). DOI https://doi.org/10.1007/s00227-017-3183-0
Dieck, T.I., 1992. North Pacific and North Atlantic digitate Laminaria species (Phaeophyta): hybridization experiments and temperature responses. Phycologia, 31, 147-163.
Dring, M.J. & Brown, F.A., 1982. Photosynthesis of intertidal brown algae during and after periods of emersion: a renewed search for physiological causes of zonation. Marine Ecology Progress Series, 8, 301-308.
Duggins, D.O., Eckman, J.E. & Sewell, A.T., 1990. Ecology of understory kelp environments. II. Effects of kelps on recruitment of benthic invertebrates. Journal of Experimental Marine Biology and Ecology, 143, 27-45.
Eckman, J.E. & Duggins, D.O., 1991. Life and death beneath macrophyte canopies: effects of understory kelps on growth rates and survival of marine, benthic suspension feeders. Oecologia, 87, 473-487.
Eckman, J.E. & Duggins, D.O., 1993. Effects of flow speed on growth of benthic suspension feeders. Biological Bulletin, 185, 28-41.
Eckman, J.E., Duggins, D.O., Sewell, A.T., 1989. Ecology of understory kelp environments. I. Effects of kelps on flow and particle transport near the bottom. Journal of Experimental Marine Biology and Ecology, 129, 173-187.
Edyvean, R.G.J. & Ford, H., 1984b. Population biology of the crustose red alga Lithophyllum incrustans Phil. 3. The effects of local environmental variables. Biological Journal of the Linnean Society, 23, 365-374.
Engelen, A.H., Serebryakova, A., Ang, P., Britton-Simmons, K., Mineur, F., Pedersen, M. F., & Toth, G., 2015. Circumglobal invasion by the brown seaweed Sargassum muticum. Oceanography and Marine Biology: An Annual Review, 53, 81-126.
Epstein, G. & Smale, D.A., 2017. Undaria pinnatifida: A case study to highlight challenges in marine invasion ecology and management. Ecology and Evolution, 7 (20), 8624-8642. DOI https://doi.org/10.1002/ece3.3430
Epstein, G. & Smale, D.A., 2018. Environmental and ecological factors influencing the spillover of the non-native kelp, Undaria pinnatifida, from marinas into natural rocky reef communities. Biological Invasions, 20 (4), 1049-1072. DOI https://doi.org/10.1007/s10530-017-1610-2
Epstein, G., Foggo, A. & Smale, D.A., 2019a. Inconspicuous impacts: Widespread marine invader causes subtle but significant changes in native macroalgal assemblages. Ecosphere, 10 (7). DOI https://doi.org/10.1002/ecs2.2814
Epstein, G., Hawkins, S.J. & Smale, D.A., 2019b. Identifying niche and fitness dissimilarities in invaded marine macroalgal canopies within the context of contemporary coexistence theory. Scientific Reports, 9. DOI https://doi.org/10.1038/s41598-019-45388-5
Ewers, R., Kasperk, C. & Simmons, B., 1987. Biologishes Knochenimplantat aus Meeresalgen. Zahnaerztliche Praxis, 38, 318-320.
Farrell, P. & Fletcher, R., 2006. An investigation of dispersal of the introduced brown alga Undaria pinnatifida (Harvey) Suringar and its competition with some species on the man-made structures of Torquay Marina (Devon, UK). Journal of Experimental Marine Biology and Ecology, 334 (2), 236-243.
Fernández, P.A., Roleda, M.Y. & Hurd, C.L., 2015. Effects of ocean acidification on the photosynthetic performance, carbonic anhydrase activity and growth of the giant kelp Macrocystis pyrifera. 124 (3), 293-304. DOI https://doi.org/10.1007/s11120-015-0138-5
Filbee-Dexter, Karen & Wernberg, Thomas, 2018. Rise of Turfs: A New Battlefront for Globally Declining Kelp Forests. BioScience, 68 (2), 64-76. DOI http://doi.org/10.1093/biosci/bix147
Fletcher, R. & Farrell, P., 1998. Introduced brown algae in the North East Atlantic, with particular respect to Undaria pinnatifida (Harvey) Suringar. Helgolander Meeresuntersuchungen, 52 (3-4), 259-275.
Forrest, B.M. & Taylor, M.D., 2002. Assessing invasion impact: Survey design considerations and implications for management of an invasive marine plant. Biological Invasions, 4 (4), 375-386. DOI https://doi.org/10.1023/A:1023613428351
Frölicher, T.L., Fischer, E.M. & Gruber, N., 2018. Marine heatwaves under global warming. Nature, 560 (7718), 360-364. DOI https://doi.org/10.1038/s41586-018-0383-9
Gayral, P. & Cosson, J., 1973. Exposé synoptique des données biologiques sur la laminaire digitée Laminaria digitata. Synopsis FAO sur les pêches, no. 89.
Gordillo, F.J.L., Aguilera, J., Wiencke, C. & Jiménez, C., 2015. Ocean acidification modulates the response of two Arctic kelps to ultraviolet radiation. Journal of Plant Physiology, 173, 41-50. DOI https://doi.org/10.1016/j.jplph.2014.09.008
- Hargrave, M.S., Foggo, A., Pessarrodona, A. & Smale, D.A., 2017. The effects of warming on the ecophysiology of two co-existing kelp species with contrasting distributions. Oecologia, 183 (2), 531-543. DOI https://doi.org/10.1007/s00442-016-3776-1
Hawkins, S.J. & Harkin, E., 1985. Preliminary canopy removal experiments in algal dominated communities low on the shore and in the shallow subtidal on the Isle of Man. Botanica Marina, 28, 223-30.
Heiser, S., Hall-Spencer, J.M. & Hiscock, K., 2014. Assessing the extent of establishment of Undaria pinnatifida in Plymouth Sound Special Area of Conservation, UK. Marine Biodiversity Records, 7, e93.
Hiscock, K., 1983. Water movement. In Sublittoral ecology. The ecology of shallow sublittoral benthos (ed. R. Earll & D.G. Erwin), pp. 58-96. Oxford: Clarendon Press.
Hoare, R. & Hiscock, K., 1974. An ecological survey of the rocky coast adjacent to the effluent of a bromine extraction plant. Estuarine and Coastal Marine Science, 2 (4), 329-348.
Holt, T.J., Jones, D.R., Hawkins, S.J. & Hartnoll, R.G., 1995. The sensitivity of marine communities to man induced change - a scoping report. Countryside Council for Wales, Bangor, Contract Science Report, no. 65.
Huthnance, J., 2010. Ocean Processes Feeder Report. London, DEFRA on behalf of the United Kingdom Marine Monitoring and Assessment Strategy (UKMMAS) Community.
Iñiguez, C., Carmona, R., Lorenzo, M.R., Niell, F.X., Wiencke, C. & Gordillo, F.J.L., 2016a. Increased CO2 modifies the carbon balance and the photosynthetic yield of two common Arctic brown seaweeds: Desmarestia aculeata and Alaria esculenta. Polar Biology, 39 (11), 1979-1991. DOI https://doi.org/10.1007/s00300-015-1724-x
Irvine, L. M. & Chamberlain, Y. M., 1994. Seaweeds of the British Isles, vol. 1. Rhodophyta, Part 2B Corallinales, Hildenbrandiales. London: Her Majesty's Stationery Office.
Jackson, A.C. & McIlvenny, J., 2011. Coastal squeeze on rocky shores in northern Scotland and some possible ecological impacts. Journal of Experimental Marine Biology and Ecology, 400 (1), 314-321. DOI https://doi.org/10.1016/j.jembe.2011.02.012
Jacobson, M.Z., 2005. Studying ocean acidification with conservative, stable numerical schemes for nonequilibrium air-ocean exchange and ocean equilibrium chemistry. Journal of Geophysical Research: Atmospheres, 110 (D7). DOI https://doi.org/10.1029/2004JD005220
James, K, 2017. A review of the impacts from invasion by the introduced kelp Undaria pinnatifida. Waikato Regional Council Technical Report 2016/40, Institute of Marine Science, University of Auckland, Hamilton, 40 pp. Available from: https://www.waikatoregion.govt.nz/assets/WRC/WRC-2019/TR201640.pdf
JNCC (Joint Nature Conservation Committee), 2022. The Marine Habitat Classification for Britain and Ireland Version 22.04. [Date accessed]. Available from: https://mhc.jncc.gov.uk/
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Jones, L.A., Hiscock, K. & Connor, D.W., 2000. Marine habitat reviews. A summary of ecological requirements and sensitivity characteristics for the conservation and management of marine SACs. Joint Nature Conservation Committee, Peterborough. (UK Marine SACs Project report.). Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/marine-habitats-review.pdf
Kain, J.M., 1975a. Algal recolonization of some cleared subtidal areas. Journal of Ecology, 63, 739-765.
Kain, J.M., 1979. A view of the genus Laminaria. Oceanography and Marine Biology: an Annual Review, 17, 101-161.
Kain, J.M., 1979. A view of the genus Laminaria. Oceanography and Marine Biology: an Annual Review, 17, 101-161.
Kain, J.M., & Norton, T.A., 1990. Marine Ecology. In Biology of the Red Algae, (ed. K.M. Cole & Sheath, R.G.). Cambridge: Cambridge University Press.
Kindig, A.C., & Littler, M.M., 1980. Growth and primary productivity of marine macrophytes exposed to domestic sewage effluents. Marine Environmental Research, 3, 81-100.
Kinne, O. (ed.), 1971a. Marine Ecology: A Comprehensive, Integrated Treatise on Life in Oceans and Coastal Waters. Vol. 1 Environmental Factors, Part 2. Chichester: John Wiley & Sons.
Kinne, O. (ed.), 1972. Marine Ecology: A Comprehensive, Integrated Treatise on Life in Oceans and Coastal Waters,Vol.1, Environmental Factors, part 3. New York: John Wiley & Sons.
Koch, M., Bowes, G., Ross, C. & Zhang, X.-H., 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology, 19 (1), 103-132. DOI https://doi.org/10.1111/j.1365-2486.2012.02791.x
Kraan, S., 2017. Undaria marching on; late arrival in the Republic of Ireland. Journal of Applied Phycology, 29 (2), 1107-1114. DOI https://doi.org/10.1007/s10811-016-0985-2
Krause-Jensen, D., Duarte, C.M., Hendriks, I.E., Meire, L., Blicher, M.E., Marbà, N. & Sejr, M.K., 2015. Macroalgae contribute to nested mosaics of pH variability in a subarctic fjord. Biogeosciences, 12 (16), 4895-4911. DOI https://doi.org/10.5194/bg-12-4895-2015
Li, Y., Zhang, H., Tang, C., Zou, T. & Jiang, D., 2016. Influence of Rising Sea Level on Tidal Dynamics in the Bohai Sea. 74 (SI), 22-31. DOI https://doi.org/10.2112/si74-003.1
Littler, M.M., & Kauker, B.J., 1984. Heterotrichy and survival strategies in the red alga Corallina officinalis L. Botanica Marina, 27, 37-44.
Lüning, K., 1990. Seaweeds: their environment, biogeography, and ecophysiology: John Wiley & Sons.
Lüning, K., 1984. Temperature tolerance and biogeography of seaweeds: the marine algal flora of Helgoland (North Sea) as an example. Helgolander Meeresuntersuchungen, 38, 305-317.
Macleod, A., Cottier-Cook, E., Hughes, D. & Allen, C., 2016. Investigating the impacts of marine invasive non-native species. Natural England Commissioned Report NECR223, Natural England, 58 pp. Available from: https://pureadmin.uhi.ac.uk/ws/portalfiles/portal/3729569/NECR223_edition_1.pdf
Markham, J.W. & Munda, I.M., 1980. Algal recolonisation in the rocky eulittoral at Helgoland, Germany. Aquatic Botany, 9, 33-71.
Melville, A. & Connell, S., 2001. Experimental effects of kelp canopies on subtidal coralline algae. Austral Ecology, 26 (1), 102-108.
Merzouk, A. & Johnson, L.E., 2011. Kelp distribution in the northwest Atlantic Ocean under a changing climate. Journal of Experimental Marine Biology and Ecology, 400 (1), 90-98.
Minchin, D. & Nunn, J., 2014. The invasive brown alga Undaria pinnatifida (Harvey) Suringar, 1873 (Laminariales: Alariaceae), spreads northwards in Europe. Bioinvasions Records, 3 (2), 57-63. DOI http://dx.doi.org/10.3391/bir.2014.3.2.01
Morgan, K.C., Shacklock, P.F. & Simpson, F.J., 1980. Some aspects of the culture of Palmaria palmata in greenhouse tanks. Botanica Marina, 23, 765-770.
- Nepper-Davidsen, J., Andersen, D.T. & Pedersen, M.F., 2019. Exposure to simulated heatwave scenarios causes long-term reductions in performance in Saccharina latissima. Marine Ecology Progress Series, 630, 25-39
Nunes, J., McCoy, S.J., Findlay, H.S., Hopkins, F.E., Kitidis, V., Queirós, A.M., Rayner, L. & Widdicombe, S., 2015. Two intertidal, non-calcifying macroalgae (Palmaria palmata and Saccharina latissima) show complex and variable responses to short-term CO2 acidification. ICES Journal of Marine Science, 73 (3), 887-896. DOI https://doi.org/10.1093/icesjms/fsv081
O'Brien, P.J. & Dixon, P.S., 1976. Effects of oils and oil components on algae: a review. British Phycological Journal, 11, 115-142.
O'Connor, R.J., Seed, R. & Boaden, P.J.S., 1979. Effects of environment and plant characteristics on the distribution of Bryozoa in a Fucus serratus L. community. Journal of Experimental Marine Biology and Ecology, 38, 151-178.
Olabarria, Celia, Rodil, Iván F., Incera, Mónica & Troncoso, Jesús S., 2009. Limited impact of Sargassum muticum on native algal assemblages from rocky intertidal shores. Marine Environmental Research, 67 (3), 153-158. DOI https://doi.org/10.1016/j.marenvres.2008.12.007
Peckol, P., Levings, S.C. & Garrity, S.D., 1990. Kelp response following the World Prodigy oil spill. Marine Pollution Bulletin, 21, 473-476.
Pickering, M.D., Wells, N.C., Horsburgh, K.J. & Green, J.A.M., 2012. The impact of future sea-level rise on the European Shelf tides. Continental Shelf Research, 35, 1-15. DOI https://doi.org/10.1016/j.csr.2011.11.011
Picton, B.E. & Costello, M.J., 1998. BioMar biotope viewer: a guide to marine habitats, fauna and flora of Britain and Ireland. [CD-ROM] Environmental Sciences Unit, Trinity College, Dublin.
Raybaud, V., Beaugrand, G., Goberville, E., Delebecq, G., Destombe, C., Valero, M., Davoult, D., Morin, P. & Gevaert, F., 2013. Decline in kelp in west Europe and climate. Plos One, 8 (6), e66044.
Read, P.A., Anderson, K.J., Matthews, J.E., Watson, P.G., Halliday, M.C. & Shiells, G.M., 1983. Effects of pollution on the benthos of the Firth of Forth. Marine Pollution Bulletin, 14, 12-16.
Robbins, J.V., 1978. Effects of physical and chemical factors on photosynthetic and respiratory rates of Palmaria palmata (Florideophyceae), In Proceedings of the ninth International Seaweed Symposium, Santa Barbara, California, USA, 20-27 August 1977, (ed. Jensen, A. & Stein, J.R.), 273-283. Science Press, Princeton, NJ, USA.
Robins, M.W., 1968. The ecology of Alcyonium species in the Scilly Isles. Report of the Underwater Association, 3, 67-71
Rogers-Bennett, L. & Catton, C.A., 2019. Marine heatwave and multiple stressors tip bull kelp forest to sea urchin barrens. Scientific Reports, 9 (1), 15050. DOI https://doi.org/10.1038/s41598-019-51114-y
Roleda, M.Y., Morris, J.N., McGraw, C.M. & Hurd, C.L., 2012. Ocean acidification and seaweed reproduction: increased CO2 ameliorates the negative effect of lowered pH on meiospore germination in the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae). Global Change Biology, 18 (3), 854-864. DOI https://doi.org/10.1111/j.1365-2486.2011.02594.x
Ryland, J.S. & Hayward, P.J. 1977. British anascan bryozoans. London: Academic Press. Synopses of the British Fauna no. 10.
Scheibling, R.E. & Gagnon, P., 2006. Competitive interactions between the invasive green alga Codium fragile ssp tomentosoides and native canopy-forming seaweeds in Nova Scotia (Canada). Marine Ecology Progress Series, 325, 1-14.
Sebens, K.P., 1986. Spatial relationships among encrusting marine organisms in the New England subtidal zone. Ecological Monographs, 56, 73-96. DOI https://doi.org/10.2307/2937271
Seed, R., O'Connor, R.J. & Boaden, P.J.S., 1983. The spatial niche of Dynamena pumila (L.) and Gonothyraea loveni (Allman) (Hydrozoa) within a Fucus serratus L. community. Cahiers de Biologie Marine, 24, 391-419.
SEEEC (Sea Empress Environmental Evaluation Committee), 1998. The environmental impact of the Sea Empress oil spill. Final Report of the Sea Empress Environmental Evaluation Committee, 135 pp., London: HMSO.
Simonson, E., Scheibling, R. & Metaxas, A., 2015. Kelp in hot water: I.Warming seawater temperature induces weakening and loss of kelp tissue. Marine Ecology Progress Series, 537. DOI http://doi.org/10.3354/meps11438
Smale, D.A., 2020. Impacts of ocean warming on kelp forest ecosystems. New Phytologist, 225, 1447-1454. DOI https://doi.org/10.1111/nph.16107
Smale, D.A., Wernberg, T., Oliver, E.C.J., Thomsen, M., Harvey, B.P., Straub, S.C., Burrows, M.T., Alexander, L.V., Benthuysen, J.A., Donat, M.G., Feng, M., Hobday, A.J., Holbrook, N.J., Perkins-Kirkpatrick, S.E., Scannell, H.A., Sen Gupta, A., Payne, B.L. & Moore, P.J., 2019. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nature Climate Change, 9 (4), 306-312. DOI https://doi.org/10.1038/s41558-019-0412-1
Smale, D.A., Wernberg, T., Yunnie, A.L. & Vance, T., 2014. The rise of Laminaria ochroleuca in the Western English Channel (UK) and comparisons with its competitor and assemblage dominant Laminaria hyperborea. Marine ecology.
Smith, B.D., 1985. Recovery following experimental harvesting of Laminaria longicruris and Laminaria digitata in Southwestern Nova Scotia. Helgolander Meeresuntersuchungen, 39(1), 83-101.
Smith, J.E. (ed.), 1968. 'Torrey Canyon'. Pollution and marine life. Cambridge: Cambridge University Press.
South, P. M., Lilley, S. A., Tait, L. W., Alestra, T., Hickford, M. J. H., Thomsen, M. S. & Schiel, D. R., 2016. Transient effects of an invasive kelp on the community structure and primary productivity of an intertidal assemblage. Marine and Freshwater Research, 67 (1), 103-112. DOI https://doi.org/10.1071/MF14211
Southward, A.J. & Southward, E.C., 1978. Recolonisation of rocky shores in Cornwall after use of toxic dispersants to clean up the Torrey Canyon spill. Journal of the Fisheries Research Board of Canada, 35, 682-706.
Staehr, P.A., Pedersen, M.F., Thomsen, M.S., Wernberg, T. & Krause-Jensen, D., 2000. Invasion of Sargassum muticum in Limfjorden (Denmark) and its possible impact on the indigenous macroalgal community. Marine Ecology Progress Series, 207, 79-88. DOI https://doi.org/10.3354/meps207079
Sundene, O., 1964. The ecology of Laminaria digitata in Norway in view of transplant experiments. Nytt Magasin for Botanik, 11, 83-107.
Teagle, H., Hawkins, S. J., Moore, P. J. & Smale, D. A., 2017. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. Journal of Experimental Marine Biology and Ecology, 492, 81-98. DOI https://doi.org/10.1016/j.jembe.2017.01.017
Thomas, J.G., 1940. Pomatoceros, Sabella and Amphitrite. LMBC Memoirs on typical British marine plants and animals no.33. University Press of Liverpool. Liverpool
Thompson, G.A. & Schiel, D.R., 2012. Resistance and facilitation by native algal communities in the invasion success of Undaria pinnatifida. Marine Ecology, Progress Series, 468, 95-105.
Thomsen, M.S., Mondardini, L., Alestra, T., Gerrity, S., Tait, L., South, P.M., Lilley, S.A. & Schiel, D.R., 2019. Local Extinction of Bull Kelp (Durvillaea spp.) Due to a Marine Heatwave. 6 (84). DOI https://doi.org/10.3389/fmars.2019.00084
Thomsen, Mads S., Wernberg, Thomas, Stæhr, Peter A. & Pedersen, Morten F., 2006. Spatio-temporal distribution patterns of the invasive macroalga Sargassum muticum within a Danish Sargassum-bed. Helgoland Marine Research, 60 (1), 50-58. DOI https://doi.org/10.1007/s10152-005-0016-1
Tidbury, H, 2020. Wakame (Undaria pinnatifida). GB Non-native Species Rapid Risk Assessment., 15 pp. Available from: http://www.nonnativespecies.org/index.cfm?pageid=143
Todd, C.D. & Lewis, J.R., 1984. Effects of low air-temperature on Laminaria digitata in Southwestern Scotland. Marine Ecology Progress Series, 16, 199-201.
Turner, S.J. & Todd, C.D., 1991. The effects of Gibbula cineraria (L.), Nucella lapillus (L.) and Asterias rubens L. on developing epifaunal assemblages. Journal of Experimental Marine Biology and Ecology, 154, 191-213.
Valentine, J. P. & Johnson, C. R., 2003. Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. Journal of Experimental Marine Biology and Ecology, 295 (1), 63-90. DOI https://doi.org/10.1016/S0022-0981(03)00272-7
Van den Hoek, C., 1982. The distribution of benthic marine algae in relation to the temperature regulation of their life histories. Biological Journal of the Linnean Society, 18, 81-144.
Van der Meer, J.P. & Chen, C-M., 1979. Evidence for sexual reproduction in the red algae Palmaria palmata and Halosaccion ramentaceum.
Vaz-Pinto, F., Rodil, I.F., Mineur, F., Olabarria, C. & Arenas, F., 2014. Understanding biological invasions by seaweeds. In Pereira, L. & Neto, J.M. (eds.). Marine algae: biodiversity, taxonomy, environmental assessment and biotechnology. Boca Raton, Florida: CRC Press, pp. 140-177.
Viejo, R.M., Arrontes, J. & Andrew, N.L., 1995. An Experimental Evaluation of the Effect of Wave Action on the Distribution of Sargassum muticum in Northern Spain. , 38 (1-6), 437-442. DOI https://doi.org/10.1515/botm.1995.38.1-6.437
- Wiens, J.J., 2016. Climate-Related Local Extinctions Are Already Widespread among Plant and Animal Species. PLOS Biology, 14 (12), e2001104. DOI https://doi.org/10.1371/journal.pbio.2001104
Wilson, K.L., Kay, L.M., Schmidt, A.L. & Lotze, H.K., 2015. Effects of increasing water temperatures on survival and growth of ecologically and economically important seaweeds in Atlantic Canada: implications for climate change. 162 (12), 2431-2444. DOI https://doi.org/10.1007/s00227-015-2769-7
Wotton, D.M., O'Brien, C., Stuart, M.D. & Fergus, D.J., 2004. Eradication success down under: heat treatment of a sunken trawler to kill the invasive seaweed Undaria pinnatifida. Marine Pollution Bulletin, 49 (9), 844-849.
Citation
This review can be cited as:
Last Updated: 10/05/2022
- tide swept
- tideswept
- kelp